Comparative study of the content and profiles of macronutrients in spelt and wheat, a review
Centre wallon de Recherches agronomiques. Département Sciences du Vivant. Unité Amélioration des Espèces et Biodiversité. Bâtiment Émile Marchal. Rue de Liroux, 4. B-5030 Gembloux (Belgium). E-mail: escarnot@cra.wallonie.be
Centre wallon de Recherches agronomiques. Département Sciences du Vivant. Unité Amélioration des Espèces et Biodiversité. Bâtiment Émile Marchal. Rue de Liroux, 4. B-5030 Gembloux (Belgium).
Centre wallon de Recherches agronomiques. Département Valorisation des Productions. Bâtiment Haute-Belgique. Rue de Serpont, 100. B-6800 Libramont (Belgium).
Univ. Liege - Gembloux Agro-Bio Tech. Unité de Chimie biologique industrielle. Passage des Déportés, 2. B- 5030 Gembloux (Belgium).
Received on March 15, 2011; accepted on December 8, 2011.
Résumé
Étude comparative des teneurs et profils des macronutriments de l'épeautre et du blé. L'épeautre (Triticum spelta) est un blé hexaploïde, vêtu, au rachis cassant qui présente des caractéristiques agronomiques d'intérêt. Il est utilisé en alimentation animale et humaine et se développe particulièrement sur le segment des produits naturels. L'épeautre se distingue du froment par ses teneurs supérieures en protéines (15,6versus 14,9 %) et lipides (2,5 versus 2,1 %) et ses teneurs inférieures en fibres insolubles (9,3 versus 11,2 %) et fibres totales (10,9 versus 14,9 %). Les teneurs en amidon, sucres et fibres solubles ne présentent pas de différences importantes. Une diversité qualitative au niveau des protéines, des arabinoxylanes et des acides gras est observée.
Abstract
Spelt (Triticum spelta) is a hexaploid wheat, hulled and with a brittle rachis, and it has interesting agronomic properties. It is used in feed and food, and is becoming more widely used in the growing natural foods market. Spelt differs from wheat in that it has a higher protein content (15.6% for spelt, 14.9% for wheat), higher lipid content (2.5% and 2.1%, respectively), lower insoluble fiber content (9.3% and 11.2%, respectively) and lower total fiber content (10.9% and 14.9%, respectively). There are no important differences in starch, sugar and soluble fiber content, and there is a qualitative diversity at the protein, arabinoxylan and fatty acid levels.
1. Introduction
1Spelt (Triticum spelta) is a hexaploid cereal belonging to the Triticum genus in the Gramineae family. Spelt grains are hulled and the hull represents 21-32% of the harvested product (Percival, 1921). Spelt spikes are pyramidal and the rachis is brittle (Luo et al., 2000). The relationship between spelt and wheat has been investigated extensively, with most studies postulating that they belong to the same species, but to separate gene pools. The literature continues to consider them as distinct species, more from the point of view of use rather than genetics (Abdel-Aal et al., 2005). The most recent studies on the phylogenic origin of spelt support the hypothesis that spelt results from several hybridizations between club wheat and a hulled tetraploid emmer (Yan et al., 2003; An et al., 2005). It has been established that spelt originated from the Middle-East and migrated northwards along the Black Sea and the Danube from East to West, reaching Austria, Southern Germany and Northern Switzerland (Andrews, 1964). In Europe, during a period of climatic cooling (750-15 BC), spelt replaced einkorn (Triticum monococcum) and emmer (Triticum dicoccum) and then, in turn, was replaced by free-threshing wheat almost throughout Europe during the first millennium (Nesbitt et al., 1996).
2In terms of agronomic characteristics, spelt displays high resistance to environmental factors such as diseases and stress, and can produce good yields under disadvantageous growing conditions such as wet, cold soils and high altitudes (Campbell, 1997). In addition, as the hull covers the seed, chemical treatment before sowing is not always necessary and, because of its long straw, spelt cannot withstand a high level of nitrogen fertilization (Bonafaccia et al., 2000). It is suitable for organic farming and contributes to agro-biodiversity, thus meeting the objectives of the European Union with regard to growing practices. Spelt is now cultivated in Europe, Asia (Iran), North Africa, the USA and Canada (Abdel-Aal et al., 1998a; Dvoracek et al., 2002). It is used mainly in animal feed in order to provide a balanced intake for animals fed primarily on grass silage (Lecomte et al., 1996). As an ancient crop, however, spelt occupies a niche market in North America and Europe in the natural, organic, health and specialty-food markets (Abdel-Aal et al., 2005). It has the potential for a variety of uses, including bread, pasta and breakfast cereals (Abdel-Aal et al., 1998b; Bonafaccia et al., 2000). These uses are similar to those associated with wheat, although the characteristics differ. The composition of the two cereals has been investigated for several decades and over the past five years a number of studies have produced more detailed information. The renewed interest in spelt requires an update on its composition. This paper discusses the macronutrients of spelt and wheat and highlights the differences between these cereals. The focus is on non-fiber carbohydrates, fibers, proteins and lipids.
2. Non-fiber carbohydrates
3Carbohydrates provide 40-75% of total energy intake, constituting the most important energy source in human diets (Gray, 2003). They are usually classified according to their degree of polymerization: sugars, oligosaccharides and polysaccharides (FAO, 1998). For both spelt and wheat, carbohydrates are the main components (59-71%) of the grain kernel (Belitz et al., 1999). Various studies have indicated that there is no great difference in total carbohydrate, starch and sugar content between spelt and wheat whole flour (Abdel-Aal et al., 1995; Ranhotra et al., 1995; Grela, 1996; Ranhotra et al., 1996) (Table 1).
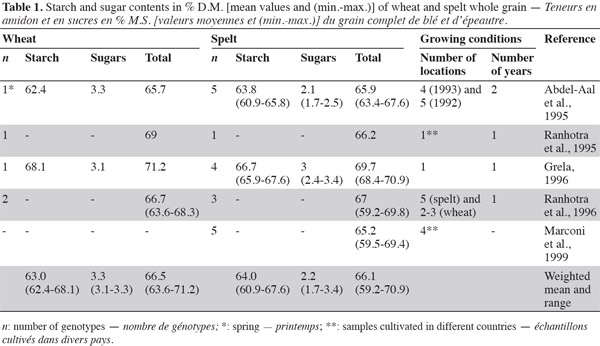
2.1. Starch
4Starch is the main storage carbohydrate in spelt and wheat kernels, accounting for 61-68% of the grain, whereas sugars account for 2-3% (Abdel-Aal et al., 2005). According to Abdel-Aal et al. (1999a) and Abdel-Aal et al. (1999b), spelt has a lower amylose content than wheat, but Wilson et al. (2008) reported a higher amylose content (2-21%) in spelt starch than in the hard red winter wheat control. Amylose and amylopectin are two components of the starch granule, accounting for 26-28% and 72-74%, respectively; amylose is a linear glucose homopolymer of glucose, and amylopectin is a branched homopolymer of glucose (Feillet, 2000).
5Gelatinization is the transformation of starch granules in four stages: loss of crystallinity (fusion of the crystalline phase, due mainly to amylopectin); water absorption (swelling); bursting of the granules; and amylose solubilization. This phenomenon is irreversible and results in increased viscosity and starch jellification when temperatures fall (Feillet, 2000). Jorgensen et al. (1997) reported that the gelatinization temperature, measured with a Brabender amylograph, was higher in spelt (87-93.2°C) than in common wheat (84.6°C) varieties. Abdel-Aal et al. (1999b) found a wide range of transition temperatures for spelt starches compared with wheat, but the difference in the enthalpy of the gelatinization of spelt starch was very similar to that of common wheat starch.
6With regard to starch size distribution, Abdel-Aal et al. (1999a) observed little difference. Wilson et al. (2008) studied the relationship between the granules and technological characteristics in spelt. Negative correlations were observed between the large A-type granules and breadcrumb score, amylose level, pasting viscosity for cultivars grown in 1999 and pasting temperature for those grown in 1998. Positive correlations were found between the small B- and C-type granules and crumb score, loaf volume, amylose, Rapid Visco Analyser (RVA) final pasting viscosity for cultivars grown in 1999 and RVA pasting temperature for those grown in 1998.
7Intra-species variability in starch content has been observed, which could be explained by the genotype and growing season conditions, as reported in Massaux et al. (2008) for wheat.
2.2. Sugars
8Sugar content in spelt samples has been found to be more variable than in wheat samples, but the number of samples that have been investigated is limited (Abdel-Aal et al., 1995; Ranhotra et al., 1995; Grela, 1996; Ranhotra et al., 1996). With regard to free sugars, there is no difference in the total concentration between spelt and modern wheat (Zorb et al., 2007).
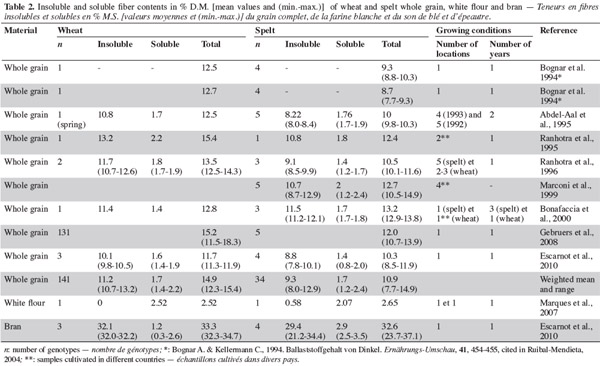
3. Fibers
9Dietary fiber has beneficial physiological effects, such as laxation. Specifically, insoluble dietary fiber reduces transit time and increases fecal bulk and defecation frequency (AACC, 2001). Dietary fiber fermentation results in the production of short-chain fatty acids conducive to bowel health (Moore et al., 1998). In addition, high dietary fiber intake reduces the risk of diverticular disease, hemorrhoids and colorectal cancer (AACC, 2001). Reduced blood cholesterol and/or blood glucose has also been attributed to dietary fiber, which is linked to a reduced risk of cardiac disease through reduced blood cholesterol and the prevention of the development of type 2 diabetes (AACC, 2001; Gray, 2006). Fibers help control body weight, mainly through inducing satiety (Gray, 2006).
3.1. Fibers in whole grain
10According to several studies (Bognar et al., 19941 cited in Ruibal-Mendieta, 2004; Abdel-Aal et al., 1995; Ranhotra et al., 1995; Ranhotra et al., 1996; Bonafaccia et al., 2000; Escarnot et al., 2010), the range of total dietary fiber content is greater in spelt than in wheat. This was not the finding in one study, however, conducted by Gebruers et al. (2008), but this could have been due to the higher number of wheat genotypes (131) considered (Table 2). Most studies have found that common wheat is richer in dietary fiber than spelt, and the same is true for insoluble fiber (Abdel-Aal et al., 1995; Ranhotra et al., 1995; Ranhotra et al., 1996; Escarnot et al., 2010). Escarnot et al. (2010) attributed this difference to hemicellulose and cellulose. For soluble fiber, the range is similar in spelt and wheat. Uniform values have been reported by most authors, including Bonafaccia et al. (2000), Abdel-Aal et al. (1995) and Ranhotra et al. (1996); Escarnot et al. (2010) found no statistical difference between spelt and wheat for soluble fiber content. Work done by Gebruers et al. (2008) shows that the total arabinoxylan content is 1.75% (1.60-2.25) for spelt and 1.90% (1.35-2.75) for wheat, and the water-extractable arabinoxylan content is 0.35% (0.30-0.45) for spelt and 0.50% (0.30-1.40) for wheat. The large range for wheat may be due to the high number of samples analyzed (131). The average arabinose/xylose ratios for total and water-extractable arabinoxylans are identical: spelt 0.60 (0.55-0.60) and 0.50 (0.45-0.55); and wheat 0.60 (0.50-0.70) and 0.50 (0.40-0.55).
11According to Marconi et al. (1999), the β-glucan content of spelt is similar to that of common wheat, with a mean value of 1.2%. Gebruers et al. (2008), however, found a higher content in wheat (0.75%) than in spelt (0.65%), with ranges of 0.55-0.70% and 0.50-0.95%, respectively, displaying a higher diversity in spelt despite the low number of genotypes (5). These values accord with those noted by Loje et al. (2003), who reported 0.7% in spelt and 0.8% in wheat (based on different years of harvest, three spelt cultivars and one wheat cultivar). The concentration of fructans, such as 1-kestose and kestotetraose, is higher in spelt than in wheat (Zorb et al., 2007).
12With regard to crude fibers, the range is wider for wheat than spelt (Grela, 1996; Jorgensen et al., 1997; Moudry et al., 1999), but no general rule can be established on content (Table 3). The two studies on hemicellulose (Grela, 1996; Escarnot et al., 2010) produced contradictory results.
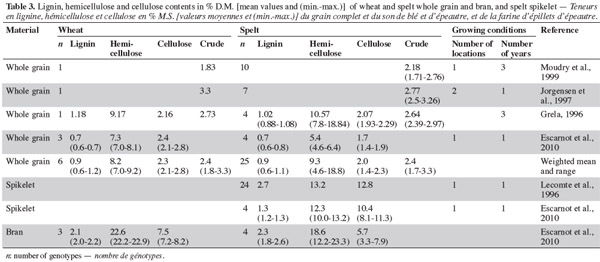
13Lignins are non-polysaccharide cell wall substances derived mainly from the three monolignols: p-coumaryl, coniferyl, and synapyl alcohols. The monolignols are targeted to different and distinct regions of various cell wall types, where they are polymerized to form wall-reinforcing biopolymers with distinctive biophysical properties (Davin et al., 2005). Lignin content is similar in spelt and wheat (Grela, 1996; Escarnot et al., 2010). The Klason lignin was evaluated by Gebruers et al. (2008), with similar levels being observed for spelt and wheat (2.25% and 2.20%, with ranges of 1.85-2.90% and 1.40-3.25%, respectively). Cellulose content is lower in spelt than in wheat (Grela, 1996; Escarnot et al., 2010).
14These studies were based on enzymatic-gravimetric methods derived from Prosky for insoluble, soluble and total fiber and from Van Soest for cellulose, hemicellulose and lignin. A minor part of the variability can therefore be attributed to the evolution of the method over the years. Intra-species variability is not surprising as it has been demonstrated that genotype and environment (including management practices), and the interaction between them, have influenced the pentosan content of wheat (Li et al., 2002; Jiang et al., 2007).
3.2. Fibers in grain milling fractions
15Escarnot et al. (2010) observed that spelt bran is richer than wheat in soluble fibers and lignin, but less rich in hemicellulose and cellulose (Table 3). In the bran, total-arabinoxylan content is much higher in wheat than in spelt (18% [13.2-22.1] and 12.7% [11.1-13.9], respectively), and the same is true for water-extractable arabinoxylan content (0.40% [0.30-0.85] and 0.30% [0.30-0.35]). The average arabinose/xylose ratio for total arabinoxylans is similar (spelt 0.50% [0.45-0.55] and wheat 0.60% [0.55-0.70]), but the average arabinose/xylose ratio for water-extractable arabinoxylans is higher for spelt (1.40% [1.20-1.6]) than for wheat (1.00% [0.70-1.65]) (Gebruers et al., 2008). Escarnot et al. (2011) extracted 55% of the arabinoxylans in spelt bran; 13% were water-extractable and 87% were water-unextractable. The populations of water-extractable arabinoxylans were 7-8 kDa and 28 kDa, and of water-unextractable arabinoxylans they were 7-8 kDa and 310-415 kDa.
16In hulled grain, Lecomte et al. (1996) reported absolute higher values of cellulose, hemicellulose and lignin, unlike those reported by Escarnot et al. (2010). The analysis of the whole spikelet flour displayed great diversity among the several genotypes studied (Escarnot et al., 2010).
17Xylanase and xylanase-inhibitors affect grain quality, production parameters and, consequently, product quality. The only study conducted on enzymatic activity (Gebruers et al., 2010) indicates that spelt white flour and bran do not display high xylanase activity. Spelt and wheat have similar Triticum aestivum xylanase inhibitor activity and display high xylanase-inhibiting protein activity. In addition, inhibitor activity is much higher in spelt bran than in spelt flour (Gebruers et al., 2010).
4. Proteins
18Proteins are a source of energy and provide essential amino acids. The wheat and spelt proteins are albumins, globulins, glutenins and gliadins. Most of the physiologically active proteins (enzymes) are albumins and globulins. They are concentrated in the cells of the aleurone layer, the pericarp and the germ, with a lower content in the endosperm. Glutenins and gliadins are storage proteins known as prolamins. They are limited to the endosperm, including the aleurone layer, and are therefore absent from the pericarp and the germ (Hoseney, 1994a).
4.1. Total content
19Most literature data indicate higher protein content in spelt than in wheat (Abdel-Aal et al., 1995; Ranhotra et al., 1995; Codianni et al., 1996; Grela, 1996; Piergiovanni et al., 1996; Ranhotra et al., 1996; Jorgensen et al., 1997; Marconi et al., 1999; Moudry et al., 1999; Bonafaccia et al., 2000; Matuz et al., 2000a; Abdel-Aal et al., 2002; Marconi et al., 2002) (Table 4). This has been confirmed under low nitrogen fertilization: Oliveira (2001) found higher protein content in spelt than in wheat, and Dvoracek et al. (2002) observed that spelt grains had 0.5% more nitrogen than common wheat grain, but this difference was not statistically significant. For white flour, Pruska-Kedzior et al. (2008) found significantly higher protein content in spelt flour (14.7%) than in common wheat flour, and Wilson et al. (2008) reached the same conclusion about spelt and a hard winter wheat control. Wilson et al. (2008) also noted highly variable protein content in spelt white flour (from five cultivars after three years of cultivation). As the degree of nitrogen absorption from the soil and its conversion into proteins depend greatly on genotype and cultivation conditions, Gräber et al. (1992) recommended comparing samples grown under the same conditions. The general attribution of high protein content in spelt could be a consequence of the low grain yield. When comparing protein yields (kg·ha-1), the values for spelt are lower than those for conventional durum wheat, with a difference of up to 25% (Piergiovanni et al., 1996). In addition, a negative heterosis effect has been observed for spelt-common wheat crosses in terms of protein content (Schmid et al., 1994).
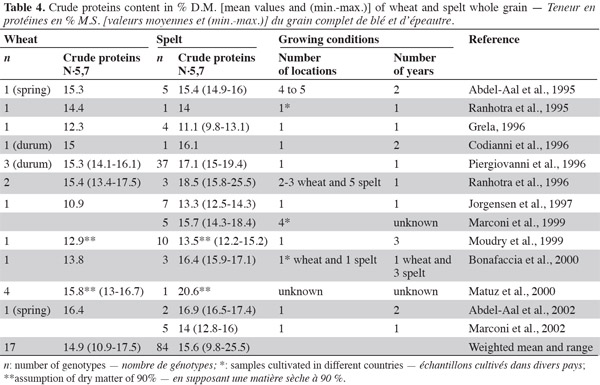
20Intra-species protein content varies widely in the different studies. All analyses were performed using the Kjeldhal method, and therefore the variability could be explained by the growing conditions (environment and nitrogen fertilization) and genetic background that influence protein content (Dupont et al., 2003).
4.2. Fractional composition and nutritional quality
21Albumins and globulins account for about 20% of the protein content in spelt (Pruska-Kedzior et al., 2008). Reversed Phase-High Performance Liquid Chromatography (RP-HPLC) has revealed a much higher content of total gliadins and a lower content of total glutenins in spelt than in wheat. The gliadin/glutenin ratio is significantly higher in spelt than in wheat (Wieser, 2000): 3.5 for spelt and 2 for common wheat (Koenig et al., 2009). In spelt, α-gliadins and γ-gliadins are predominant, whereas low and high molecular weight (LMW and HMW) glutenin subunits and ω-gliadins are generally minor components (Wieser, 2000). Acetic acid soluble prolamins accounted for 94.1% of total gluten protein in spelt flours and 85-87% of the total gluten protein in wheat flours (Pruska-Kedzior et al., 2008).
22Gliadins and glutenins in spelt differ in structure from those in common wheat (Abdel-Aal et al., 1996; Harsch et al., 1997; Radic et al., 1997; Von Büren et al., 2000) and spelt storage proteins form gluten in which the properties and quality differ from those of common wheat (Abdel-Aal et al., 1995; Schober et al., 2002).
23The in vitro digestibility of spelt and wheat proteins is similar (86.7% on average), but it is 97.6% for casein (Abdel-Aal et al., 2002). Ranhotra et al. (1995) observed a protein digestibility of 80.1% for spelt, 78.9% for wheat and 91.6% for casein, and concluded that this could suggest that spelt grain is better digested than common wheat, but the differences are minor.
24Gliadins. Through hydration, gliadins form the gluten network that makes wheat and spelt unique bread-making cereals (Hoseney, 1994b). The MW of spelt gliadins is between 34 and 75 kDa (Abdel-Aal et al., 1996). Abdel-Aal et al. (1996) and Harsch et al. (1997) found that the gliadin profile for common wheat and spelt differed. Spelt gliadins do not have slow-moving ω-gliadin or strong-staining fast-moving ω-gliadin, but these are present in common wheat. Spring and winter spelt were characterized by a large number of slow-moving α-gliadins. A γ-gliadin band was also observed in spring spelt, but not in winter spelt or wheat, and this could be a useful point of distinction. Von Büren et al. (2000) discovered an unknown γ-gliadin gene in 18 spelt and spelt-wheat crosses and in the cultivar Chinese Spring, whereas the 16 wheat cultivars had a previously documented allele. In 2001, Von Büren et al. (2001) developed a polymerase chain reaction (PCR)-based method on the allelic difference in the γ-gliadin gene GAG56D to determine the proportion of wheat in spelt flour and products, with a minimal detection level of 5%. Piergiovanni et al. (2003) found that lines belonging to the same species could be differentiated mainly by comparing the pattern of β- and ω-gliadins. Federmann et al. (1992) reached the same conclusion and recommended slow-moving ω-gliadins for discriminating spelt flour. Using RP-HPLC and sodium dodecyl sulfate (SDS) electrophoresis, Koenig et al. (2009) observed that spelt was deficient in the so-called ω-bound gliadins (a minor portion of the ω-gliadins with a MW of 50-55 kDa) that are present in the glutenin fraction due to one cysteine residue in the amino acid sequence. This group of proteins could therefore be used to detect and quantify small amounts of common wheat in spelt and spelt products.
25Glutenins. Most of the LMW-glutenin subunits of spelt seem acetic acid soluble. Spelt flour has been found to contain half as many NaOH-soluble glutenins (5.1% of total gluten protein) as common wheat flour (about 10% of total gluten proteins) (Pruska-Kedzior et al., 2008). HMW and LMW glutenins have been associated with bread and pasta-making quality in common and durum wheat, respectively (Pogna et al., 1990; Shewry et al., 1995). From a cross between Triticum aestivum and Triticum speltoides, Moonen et al. (1985) found that two HMW glutenin subunits (5 and 9) of common wheat coded by a gene locus on the arm of chromosome 1B (Glu-B1c) are associated with good baking quality and that the replacement of these subunits by two others derived from T. speltoides (S1 and S2) led to poorer quality in the backcrossed lines examined. Radic et al. (1997) found differences in glutenin between spelt and common wheat using SDS polyacrylamide gel on 28 spelt samples, 16 spelt-wheat crosses and 10 winter wheat samples. Radic-Miehle et al. (1998) studied SDS soluble protein with and without a pre-extraction method, where typical bands could be identified for each species. Caballero et al. (2004c) showed that variability in the LMW-glutenin subunits in spelt is higher than in other species. Alleles Glu-A3h and Glu-B3d coding LMW-glutenin subunits are present in spelt, but rare or absent in common wheat (Yan et al., 2003).
26Amino acids. Generally, cereal proteins are known for their low essential amino acid content, especially lysine (the first most deficient amino acid) (Kies et al., 1970) and threonine (the second most deficient amino acid), but they are rich in glutamic acid and proline, the major functional amino acid in dough formation (Abdel-Aal et al., 2005). Spelt contains 38.2% of essential amino acids, as does wheat (Grela, 1996); the percentage of essential amino acids over total amino acids in protein is similar for wheat and spelt, indicating equivalent protein quality (Grela, 1996; Jorgensen et al., 1997; Abdel-Aal et al., 2002; Ruibal-Mendieta, 2004) (Table 5) and equivalent biological value of proteins (indicated by the ratio of amino acid over protein) (Matuz et al., 2000a).
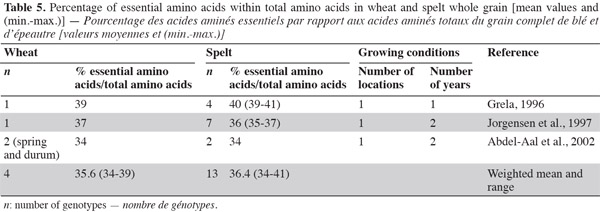
27The spelt amino acid composition of proteins differs slightly from that of wheat (Ranhotra et al., 1995; Cubadda et al., 1996; Grela, 1996; Bonafaccia et al., 2000; Abdel-Aal et al., 2002). Even if there is no statistical difference between spelt and common wheat in terms of amino acid content, there is evidence of higher values (except for isoleucine, leucine and glycine) in spelt than in common wheat (Dvoracek et al., 2002). This was confirmed by Matuz et al. (2000a), who observed that wholemeal and flour from spelt had a higher content of most amino acids than some recently developed common wheat.
28According to Grela (1996), the average lysine content is considerably higher in spelt (3.19 g per 16 gN) than in wheat (2.91 g per 16 gN), but most studies show that lysine content is lower in spelt than in wheat (Matuz et al., 2000a; Abdel-Aal et al., 2002), with a difference of up to 28% (Ranhotra et al., 1995). Jorgensen et al. (1997) reported that lysine content was 2.72 g per 16 gN (2.58-2.89) in spelt and 2.97 g per 16 gN in wheat. Clamot (1984) found considerable genetic differences in protein and lysine content among 164 spelt samples, including 77 breeding lines from old Belgian landraces, 72 introductions from various countries and 15 induced mutants over a 3-year period.
29Two studies found a higher methionine content in spelt than in common wheat (Ranhotra et al., 1995; Bonafaccia et al., 2000), but Matuz et al. (2000a) found the opposite. Jorgensen et al. (1997) found no significant differences for methionine content among the spelt varieties.
30Within the group of essential amino acids, no significant differences were found for isoleucine, leucine, phenylalanine and valine content among the spelt varieties. In the group of non-essential amino acids, spelt had significantly more proline and less alanine and arginine than wheat (Jorgensen et al., 1997). Spelt had also significantly more glutamic acid, significantly and negatively correlated with lysine (Jorgensen et al., 1997; Abdel-Aal et al., 2002) and more tyrosine than wheat (Ranhotra et al., 1995; Jorgensen et al., 1997). Aspartic acid was higher in spelt than in wheat (Ranhotra et al., 1995).
5. Lipids
5.1. Fatty acid content and profiles
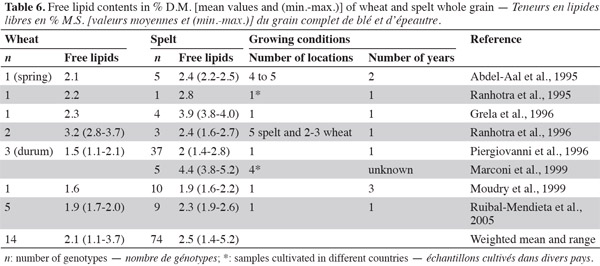
31Lipids are minor grain constituents, accounting for about 3% of the wheat kernel. They are more concentrated in the germ (which contains 28.5% of lipids) and in the aleurone layer (8.0%) than in the endosperm (1.5%) (Delcour et al., 2010). Whole-wheat lipids are made up of about 70% non-polar lipids, 20% glycolipids and 10% phospholipids (Delcour et al., 2010), to which small percentages of sterols, tocopherols and other fat-soluble vitamins are added (Abdel-Aal et al., 2005). In flour from the starchy endosperm, some lipids are associated with starch granules (1.0%), but others are not (1.4%). Among the non-starch lipids, free lipids (with non-polar and polar lipids) and bound lipids are distinct, and among the starch lipids the non-polar and polar lipids are separated (Chung et al., 2009). Starch lipids are made up of 9% non-polar lipids, 5% glycolipids and 86% phospholipids (Delcour et al., 2010). Starch lipids are contained within starch granules as inclusion complexes and located between amylose and monoacyl lipids, such as lysophosphatidylcholines (Feillet, 2000). Non-starch lipids are made up of 60% non-polar lipids, 25% glycolipids and 15% phospholipids (Delcour et al., 2010). Non-starch lipids are dispersed inside the albumen and can react with other flour constituents. In the germ and the aleurone layer, lipids are assembled into spherosomes that are triglycerides surrounded by polar lipids and proteins (Feillet, 2000). In cereals, lipid content is usually determined by extraction with non-polar solvents. Bound lipids (to starch) are not taken into account because their extraction requires the use of polar solvents. Free lipid content is a practical estimate of total content and allows the comparison of data from different laboratories (Chung et al., 2000).
32Most studies on free lipids show that spelt is richer in lipids than wheat (Abdel-Aal et al., 1995; Ranhotra et al., 1995; Grela, 1996; Piergiovanni et al., 1996; Ranhotra et al., 1996; Moudry et al., 1999; Ruibal-Mendieta et al., 2002; Ruibal-Mendieta et al., 2005) (Table 6). Ruibal-Mendieta et al. (2002) found that total lipid content was also higher for spelt than wheat and true spelt might contain more lipids than hybrid spelt, although this difference was significant in two out of three harvest years. These observations suggest that germ is present in higher proportions in spelt kernels than in common wheat kernels (Marconi et al., 1999).
33With regard to fatty acids, studies show that the major fatty acids in spelt and wheat wholemeal are linoleic, palmitic, oleic and linolenic acids (Grela, 1996; Ruibal-Mendieta et al., 2004b; Ruibal-Mendieta et al., 2005). The proportion of oleic acid in fatty acids is higher in spelt than in common wheat, but the proportion of linoleic and linolenic acids are lower in spelt than in common wheat. More saturated fatty acids have been observed in wheat than in spelt (averages of 19.8% and 18.9%, respectively; table 7).
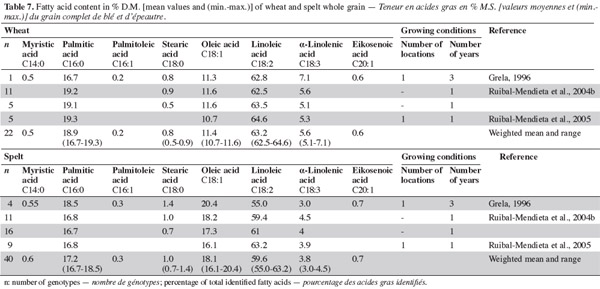
34Most results have been obtained using extraction with ether or petrolether, so the variation in content can be attributed partly to the change of method. However, it is known that lipid content and composition are influenced by genetic variation (including wheat class and cultivar), environmental effects during growth (including location, year, weather and soils) and the effects of the genetic x environment interaction (Chung et al., 2009). For fatty acids, Ruibal-Mendieta et al. (2004b), Ruibal-Mendieta et al. (2005) and Grela (1996) used different methods, respectively from Stoldt (1952) and from Folch et al. (1957) but there was variability among studies that used the same method. Method, environment, genotype and their interaction therefore all contribute to the variability.
5.2. Sterols
35Phytosterols are known to reduce serum cholesterol and could offer protection against several cancer types (Nurmi et al., 2008). The range in phytosterol content in 16 spelt genotypes (9 cultivars and 7 landraces) was broader in a study conducted by Ruibal-Mendieta et al. (2004a) than for 5 cultivars in a study by Nurmi et al. (2008). Ruibal-Mendieta et al. (2004a) observed no difference in sterol content in spelt and winter wheat, but Nurmi et al. (2008) found higher average content in spelt than in wheat (928 [893-963] µg·g-1 [D.M.] and 841 [670-959] µg·g-1 [D.M.]). This latter finding was confirmed by Iafelice et al. (2009), who reported 717 (628-819) µg·g-1 (D.M.) total sterol, on average, for spelt, and 634 (600-677) µg·g-1 (D.M.) for wheat. The free sterol content, however, was higher in wheat 324 (288-387) µg·g-1 (D.M.) than in spelt 252 (191-294) µg·g-1 (D.M.), and esterified sterol content was slightly higher in spelt 267 (251-291) µg·g-1 (D.M.) than in wheat 258 (219-330) µg·g-1 (D.M.) (Iafelice et al., 2009). This accords with the amount of free and esterified sterols measured by Ruibal-Mendieta et al. (2004a) of 527 µg·g-1 (D.M.) in spelt and of 528 µg·g-1 (D.M.) in wheat.
36Ruibal-Mendieta et al. (2004a) found that spelt and wheat display a similar sterol profile and content. The ∆7- avenasterol content is an exception; it is 45% higher in spelt than in wheat (Ruibal-Mendieta et al., 2004a). For spelt, Ruibal-Mendieta et al. (2004a) reported that sitosterol and campesterol accounted for about 70 and 20% of the total sterols, respectively, and stanols for 5%, which differed from the results reported by Nurmi et al. (2008) where sitosterol, campesterol and stanols accounted for 49%, 14% and 26%, respectively. In the Nurmi et al. (2008) study, the proportions for wheat were sitosterol 52%, campesterol 15% and stanols 24%. These differences in sterol profiles appear to have resulted from analytical differences (Nurmi et al., 2008). The results reported by Iafelice et al. (2009) confirmed the earlier ones, with 59% sitosterol, 18% campesterol and 17% stanols in wheat and 56%, 16% and 19% in spelt, respectively, among the total sterols. For the free sterols, the proportions were similar in spelt and wheat, with slightly more sitosterol (by 2%) in wheat. For the esterified sterols, the proportion of sitosterol was higher in spelt than in wheat (59% and 56%, respectively), but that of campesterol and sitostanol was lower in spelt (16% and 15%, respectively) than in wheat (18% and 17%, respectively) (Iafelice et al., 2009).
6. Conclusion
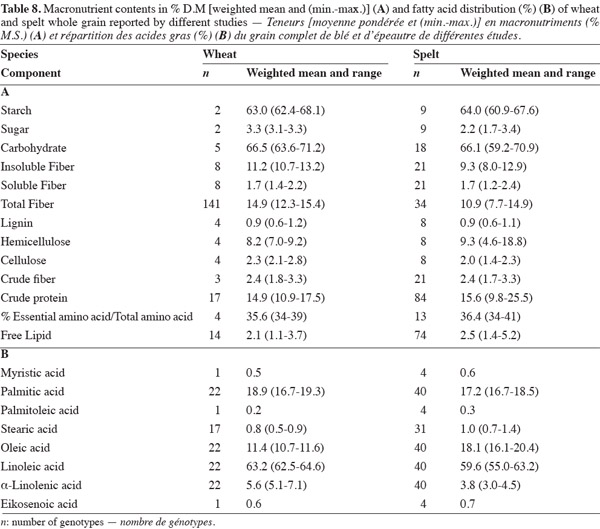
37This paper has highlighted differences in the macronutrient content and profiles of spelt and wheat. It has shown that spelt has a higher protein and lipid content and a lower insoluble and total fiber content than wheat, and that there is no significant difference between them in starch, sugar and soluble fiber content (Table 8). The differences can affect the techno-functional properties of spelt. Bread-making and pasta making from spelt requires adapted techniques, and the evaluation procedures used for wheat and wheat products should not be directly applied to spelt and spelt products (Abdel-Aal et al., 2005). This study should be followed by one on micronutrient content – ashes, minerals, vitamins and bioactive compounds – for which differences between spelt and wheat have also been reported. In addition, research on the applications of spelt should continue to look for new ways of using this crop in order to sustain its development. Exhaustive biochemical, nutritional and clinical research should be undertaken to assess claims for the pro-health properties of spelt grain and products that have not yet been scientifically proved.
38Abbreviations
39HMW: High Molecular Weight
40LMW: Low Molecular Weight
41PCR: Polymerase Chain Reaction
42RP-HPLC: Reversed Phase-High Performance Liquid Chromatography
43RVA: Rapid Visco Analyser
44SDS: Sodium Dodecyl Sulfate
Bibliographie
AACC (American Association of Cereal Chemists), 2001. The definition of dietary fiber. Cereal Foods World, 46, 112-126.
Abdel-Aal E.S.M., Hucl P. & Sosulski F.W., 1995. Compositional and nutritional characteristics of spring einkorn and spelt wheats. Cereal Chem., 72, 621-624.
Abdel-Aal E.S.M. et al., 1996. Electrophoretic characterization of spring spelt wheat gliadins. J. Agric. Food Chem., 44, 2117-2123.
Abdel-Aal E.S.M., Solsulski F.W. & Hucl P., 1998a. Origins, characteristics and potentials of ancient wheats. Cereal Food World, 43, 708-715.
Abdel-Aal E.S.M., Hucl P. & Sosulski F.W., 1998b. Food uses for ancient wheats. Cereal Food World, 43, 763-767.
Abdel-Aal E.S.M., Hucl P. & Sosulski F.W., 1999a. Optimizing the bread formulation for soft spelt wheat. Cereal Food World, 44(7), 480-483.
Abdel-Aal E.S.M., Hucl P. & Sosulski F.W., 1999b. Starches from primitive wheats. II. Thermal and structural properties. Annual meeting Abstract 225, AACC International St Paul, MN, USA, http://www.aaccnet.org/meetings/99mtg/abstracts/acabc27.htm, (05/11/2010).
Abdel-Aal E.S.M. & Hucl P., 2002. Amino acid composition and in vitro protein digestibility of selected ancient wheats and their end products. J. Food Compos. Anal., 15, 737-747.
Abdel-Aal E.S.M. & Hucl P., 2005. Spelt: a specialty wheat for emerging food uses. In: Abdel-Aal E.S.M. & Wood P. Specialty grains for food and feed. St Paul, MN, USA: American Association of Cereal Chemists, 109-141.
An X.L. et al., 2005. Genetic diversity of European spelt wheat (Triticum aestivum ssp. spelta L. em. Thell.) revealed by glutenin subunit variations at the Glu-A and Glu-3 loci. Euphytica, 146(3), 193-201.
Andrews A.C., 1964. The genetic origin of spelt and related wheats. Der Zuchter: Z. Theor. Angew. Genet., 34(1), 17-22.
Belitz H.-D. & Grosch W., 1999. Food chemistry. 2nd ed. Berlin, Heidelberg, Germany: Springer-Verlag, 631-636.
Bonafaccia G. et al., 2000. Characteristics of spelt wheat products and nutritional value of spelt wheat-based bread. Food Chem., 68, 437-441.
Caballero L., Martin L.M. & Alvarez J.B., 2004c. Genetic variability of the low-molecular-weight glutenin subunits in spelt wheat (Triticum aestivum ssp. spelta L. em. Thell.). Theor. Appl. Genet., 108, 914-919.
Campbell K.G., 1997. Spelt: agronomy, genetics and breeding. Plant Breed. Rev., 15, 187-213.
Chung O.K. & Ohm J.-B., 2000. Cereal lipids. In: Kulp K. & Ponte J.G. Jr. Handbook of cereal science and technology. 2nd ed. New York, USA: Marcel Dekker, 417-477.
Chung O.K. et al., 2009. Wheat lipids in wheat chemistry and technology. 4th ed. St Paul, MN, USA: : American Association of Cereal Chemists, 363-399.
Clamot G., 1984. Genetic variability of the protein and lysine content of spelt (Triticum spelta). Z. Pflanzenzücht., 93, 106-114.
Codianni P., Ronga G., Di Fonzo N. & Troccoli A., 1996. Performance of selected strains of 'Farro' (Triticum monococcum L., Triticum dicoccum Schübler, Triticum spelta L.) and durum wheat (Triticum durum Desf. cv. 'Trinakria') in the difficult flat environment of Southern Italy. J. Agron. Crop Sci., 176, 15-21.
Cubadda R. & Marconi E., 1996. Technological and nutritional aspects in emmer and spelt. In: Paludosi S., Hammer K. & Heller J. Hulled Wheats. Proceedings of the 1st International Workshop on hulled Wheats, 21-22 July 1995, Castelvechio Pascoli, Italy. Roma: IPGRI, 203-212.
Davin L.B. & Lewis N.G., 2005. Lignin primary structures and dirigent sites. Curr. Opin. Biotechnol., 16, 407-415.
Delcour J.A. & Hoseney R.C., 2010. Principles of cereal science and technology. 3rd ed. St Paul, MN, USA: American Association of Cereal Chemists.
Dupont F.M. & Alltenbach S.B., 2003. Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis. J. Cereal Sci., 38, 133-146.
Dvoracek V., Curn V. & Moudry J., 2002. Evaluation of amino acid content and composition in spelt wheat varieties. Cereal Res. Commun., 30, 187-193.
Escarnot E., Agneessens R., Wathelet B. & Paquot M., 2010. Quantitative and qualitative study of spelt and wheat fibres in varying milling fractions. Food Chem., 122, 857-863.
Escarnot E. et al., 2011. Extraction and characterization of water-extractable and water-unextractable arabinoxylans from spelt bran: study of the hydrolysis conditions for monosaccharides analysis. J. Cereal Sci., 53, 45-52.
FAO, 1998. Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation, 14-18 April 1997, Roma, Italy. FAO Food and nutrition paper – 66. Reprinted 1998, http://www.fao.org/docrep/W8079E/W8079E00.htm (11/01/07).
Federmann G.R., Goecke E.U. & Steiner A.M., 1992. Research note: detection of adulteration of flour spelt (Triticum spelta L.) with flour wheat (Triticum aestivum L. emend, Fiori and Paol.) by electrophoresis. Plant Var. Seeds, 5, 123-125.
Feillet P., 2000. Le grain de blé, composition et utilisation. Paris : Institut National de la Recherche Agronomique.
Folch J., Lees M. & Stanley G.H.S., 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biochem. Chem., 226, 497-509.
Gebruers K. et al., 2008. Variation in the content of dietary fiber and components thereof in wheats in the healthgrain diversity screen. J. Agric. Food Chem., 56, 9740-9749.
Gebruers K. et al., 2010. Variability in xylanase and xylanase inhibition activities in different cereals in the health grain diversity screen and contribution of environment and genotype to this variability in common wheat. J. Agric. Food Chem., 58, 9362-9371.
Gräber S. & Kuhn M., 1992. Evaluation of the baking quality of different varieties of spelt. Getreide Mehl Brot, 46, 102-108.
Gray J., 2003. Carbohydrates: nutritional and health aspects. ILSI Europe concise monograph series. Brussels: ILSI, 10.
Gray J., 2006. Dietary fibre. Definition and analysis, physiology, health. ILSI Europe concise monograph series. Brussels: ILSI, 24-31.
Grela E.R., 1996. Nutrient composition and content of antinutritional factors in spelt (Triticum spelta L.) cultivars. J. Sci. Food Agric., 71, 399-404.
Harsch S. et al., 1997. Characterization of spelt (Triticum spelta L.) forms by gel electrophoretic analyses of seed storage proteins. I. The gliadins. Theor. Appl. Genet., 94, 52-60.
Hoseney C.R., 1994a. Proteins of cereals. In: Principles of cereal science and technology. 2nd ed. St Paul, MN, USA: American Association of Cereal Chemists, 65-79.
Hoseney C.R., 1994b. Gluten proteins. In: Principles of cereal science and technology. 2nd ed. St Paul, MN, USA: American Association of Cereal Chemists, 197-211.
Iafelice G., Verardo V., Marconi E. & Caboni M.F., 2009. Characterization of total, free and esterified phytosterols in tetraploid and hexaploid wheats. J. Agric. Food Chem., 57, 2267-2273.
Jiang L.-N., Shao M., Liu L. & Li C.-X., 2007. Effects of genotypes and environments on pentosan content and viscosity in wheat grains (in Chinese/English abstract). J. Henan Agric. Sci., 1, 17-19
Jorgensen J.R., Olsen C.C. & Christiansen S., 1997. Cultivation and quality assessment of spelt (Triticum spelta L.) compared with winter wheat (Triticum aestivum L.). In: Stolen O., Bruhn K., Pithan K. & Hill J., eds. Small grain cereals and pseudo-cereals, Workshop COST 814, 22-24 February 1996, Royal Veterinary and Agricultural University, Copenhagen, Denmark. Luxembourg: Office for Official Publications of the European Communities, 31-37.
Kies C. & Fox H.M., 1970. Determination of the first limiting amino acid of wheat and triticale grain for humans. Cereal Chem., 47, 615-625.
Koenig A., Wieser H. & Koehler P., 2009. Distinguishing wheat and spelt using typical protein markers. In: Proceedings of the 10th International Gluten Workshop, 7-9 September 2009, Clermont-Ferrand, France, 142-145.
Lecomte P. et al., 1996. Caractérisation de la composition chimique et de la valeur de l'épeautre (Triticum spelta) en alimentation des ruminants. Renc. Rech. Ruminants, 3, 112.
Li C.-X., Qiu Z.-B., Jiang L.-N. & Zhang X., 2002. Research on content of pentosans in wheat grain in different ecological environment (in Chinese/English abstract). Acta Agric. Boreali Sin., 17, 1-4.
Loje H., Moller B., Laustsen A.M. & Hansen A., 2003. Chemical composition, functional properties and sensory profiling of einkorn (Triticum monococcum L.). J. Cereal Sci., 37, 231-240.
Luo M.C., Yang Z.L. & Dvorak J., 2000. The Q locus of Iranian and European spelt wheat. Theor. Appl. Genet., 100, 602-606.
Marconi E., Carcea M., Graziano M. & Cubadda R., 1999. Kernel properties and pasta-making quality of five European spelt wheat (Triticum spelta L.) cultivars. Cereal Chem., 76(1), 25-29.
Marconi E., Carcea M., Schiavone M. & Cubadda R., 2002. Spelt (Triticum spelta L.) pasta quality: combined effect of flour properties and drying conditions. Cereal Chem., 79(5), 634-639.
Massaux C. et al., 2008. Variations in physicochemical and functional properties of starches extracted from European soft wheat (Triticum aestivum L.): the importance to preserve the varietal identity. Carbohydr. Polym., 71, 32-41.
Matuz J., Bartok T., Morocz-Salamon K. & Bona L., 2000a. Structure and potential allergenic character of cereal proteins. I. Protein content and amino acid composition. Cereal Res. Commun., 28(3), 263-270.
Moonen J.H.E & Zeven A.C., 1985. Association between high molecular weight subunits of glutenin and bread-making quality in wheat lines derived from backcrosses between T. aestivum and T. speltoides. J. Cereal Sci., 3, 97-101.
Moore M.A., Beom Park C. & Tsuda H., 1998. Soluble and insoluble fiber influences on cancer development. Crit. Rev. Oncol. Hematol., 27, 229-242.
Moudry J. & Dvoracek V., 1999. Chemical composition of grain of different spelt (Triticum spelta L.) varieties. Rostlinna Vyroba, 45(12), 533-538.
Muramatsu M., 1963. Dosage effect of the spelta gene q of hexaploid wheat. Genetics, 48, 469-482.
Nesbitt M. & Samuel D., 1996. From staple crop to extinction? The archaeology and history of the hulled wheats. In: Paludosi S., Hammer K. & Heller J. Hulled Wheats. Proceedings of the 1st International Workshop on hulled Wheats, 21-22 July 1995, Castelvechio Pascoli, Italy. Roma: IPGRI, 41-100.
Nurmi T. et al., 2008. Phytosterols in wheat genotypes in the health grain diversity screen. J. Agric. Food Chem., 56, 9710-9715.
Oliveira J.A., 2001. North Spanish emmer and spelt wheat landraces: agronomical and grain quality characteristics evaluation. Plant Genet. Res. Newsl., 125, 16-20.
Percival J., 1921. The wheat plant. A monograph. London: Duckworth and Co., 325-334.
Piergiovanni A.R., Laghetti G. & Perrino P., 1996. Characteristics of meal from hulled wheats (Triticum dicoccon Schrank and T. spelta L.): an evaluation of selected accessions. Cereal Chem., 73(6), 732-735.
Piergiovanni A.R. & Volpe N., 2003. Capillary electrophoresis of gliadins as a tool in the discrimination and characterization of hulled wheats (Triticum dicoccon Schrank and T. spelta L.). Cereal Chem., 80, 269-273.
Pogna N.F. et al., 1990. Chromosome 1B-encoded gliadins and glutenins subunits in durum wheat: genetics and relationship to gluten strength. J. Cereal Sci., 11, 15-34.
Pruska-Kedzior A., Kedzior Z. & Klockiewicz-Kaminska E., 2008. Comparison of viscoelastic properties of gluten from spelt and common wheat. Eur. Food Res. Technol., 227, 199-207.
Radic H., Günther T., Kling Ch.I. & Hesemann C.U., 1997. Characterization of spelt (Triticum spelta L.) forms by gel electrophoretic analyses of seed storage proteins. II. The glutenins. Theor. Appl. Genet., 94, 882-886.
Radic-Miehle H. et al., 1998. Characterization of spelt (Triticum spelta L.) forms by gel-electrophoretic analyses of seed storage proteins. III. Comparative analyses of spelt and central European winter wheat (Triticum aestivum L.) cultivars by SDS-PAGE and acid-PAGE. Theor. Appl. Genet., 97, 1340-1346.
Ranhotra G.S., Gelroth J.A., Glaser B.K. & Lorenz K.J., 1995. Baking and nutritional qualities of spelt wheat sample. Lebensm.-Wiss. Technol., 28, 118-122.
Ranhotra G.S., Gelroth J.A., Glaser B.K. & Stallknecht G.F., 1996. Nutritional profile of three spelt wheat cultivars grown at five different locations. Cereal Chem., 73(5), 533-535.
Ruibal-Mendieta N.L., 2004. Lipids and minerals in spelt (Triticum aestivum ssp. spelta) and common wheat (T. aestivum ssp. vulgare). Chemical and nutritional distinction between both cereals. PhD thesis: Faculté d'Ingénierie biologique, agronomique et environnementale, Université catholique de Louvain (Belgium).
Ruibal-Mendieta N.L., Delacroix D.L. & Meurens M., 2002. A comparative of analysis of free, bound and total lipid content on spelt and winter wheat wholemeal. J. Cereal Sci., 35, 337-342.
Ruibal-Mendieta N.L. et al., 2004a. Spelt (Triticum spelta L.) and winter wheat (Triticum aestivum L.) wholemeals have similar sterols profiles, as determined by quantitative liquid chromatography and mass spectrometry analysis. J. Agric. Food Chem., 52, 4802-4807.
Ruibal-Mendieta N.L. et al., 2004b. The oleate/palmitate ratio allows the distinction between wholemeals of spelt (Triticum spelta L.) and winter wheat (T. aestivum L.). J. Cereal Sci., 39, 413-415.
Ruibal-Mendieta N.L. et al., 2005. Spelt (Triticum aestivum ssp. spelta) as a source of breadmaking flours and bran naturally enriched in oleic acid and minerals but not phytic acid. J. Agric. Food Chem., 53, 2751-2759.
Schmid J.E., Winzeler M. & Winzeler H., 1994. Analysis of disease resistance and quality characters of F1 hybrids of crosses between wheat (Triticum aestivum L.) and spelt (Triticum spelta L.). Euphytica, 75, 105-110.
Schober T.J., Clarke C.I. & Kuhn M., 2002. Characterization of functional properties of gluten proteins in spelt cultivars using rheological and quality factor measurements. Cereal Chem., 79, 408-417.
Shewry P.R. et al., 1995. Biotechnology of breadmaking: unraveling and manipulating the multi-protein gluten complex. Bio/Technology, 13, 1185-1190.
Stoldt W., 1952. Vorschlag zur Vereinheitlichung der Fettbestimmung in Lebensmitteln. Fette Seifen, 54, 206-207.
Von Büren M., Lüthy J. & Hübner P., 2000. A spelt-specific -gliadin gene: discovery and detection. Theor. Appl. Genet., 100, 271-279.
Von Büren M., Stadler M. & Lüthy J., 2001. Detection of wheat adulteration of spelt flour and products by PCR. Eur. Food Res. Technol., 212, 234-239.
Wieser H., 2000. Comparative investigations of gluten proteins from different wheat species. Eur. Food Res. Technol., 211, 262-268.
Wilson J.D., Bechtel D.B., Wilson G.W.T. & Seib P.A., 2008. Bread quality of spelt wheat and its starch. Cereal Chem., 85(5), 629-638.
Yan Y. et al., 2003. HMW and LMW glutenins alleles among putative tetraploid and hexaploid European spelt wheat (Triticum spelta L.) progenitors. Theor. Appl. Genet., 107, 1321-1330.
Zorb C. et al., 2007. Free sugars in spelt wholemeal and flour. J. Appl. Bot. Food Qual., 81, 172-174.
