Milk fat globule membrane and buttermilks: from composition to valorization
Univ. Liege - Gembloux Agro-Bio Tech. Department of Food Technology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Univ. Liege - Gembloux Agro-Bio Tech. Department of Food Technology. Passage des Déportés, 2. B-5030 Gembloux (Belgium) – Univ. Liege - Gembloux Agro-Bio Tech. Department of Industrial Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Univ. Liege - Gembloux Agro-Bio Tech. Department of Food Technology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Univ. Liege - Gembloux Agro-Bio Tech. Department of Industrial Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Univ. Liege - Gembloux Agro-Bio Tech. Department of Food Technology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Univ. Liege - Gembloux Agro-Bio Tech. Department of Food Technology. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: christophe.blecker@ulg.ac.be
Received on March 12, 2009; accepted on September 11, 2009
Résumé
La membrane du globule gras du lait et les babeurres : de leur composition à leur valorisation. Le babeurre est un co-produit de l'industrie beurrière trop longtemps négligé, bien qu'il soit peu couteux et disponible en grande quantité. Depuis ces vingt dernières années, un regain d'intérêt lui est toutefois porté en raison de sa composition très spécifique en protéines et en lipides polaires originaires de la membrane du globule gras du lait (MFGM). L'objectif de cette synthèse bibliographique est de faire le point sur les connaissances récentes relatives au babeurre. Premièrement, la composition et la structure de la MFGM sont décrites. Deuxièmement, des définitions du babeurre et des produits associés sont présentées en ce qui concerne le mode d'obtention de ceux-ci. La structure et la composition moyenne de ces produits sont résumées et associées aux propriétés technologiques, tout particulièrement aux propriétés émulsifiantes des composants de la MFGM. Finalement, de nouvelles applications sont présentées afin de promouvoir les valeurs ajoutées du babeurre et de ses produits dérivés.
Abstract
Buttermilk, the by-product from butter manufacture, is low cost and available in large quantities but has been considered for many years as invaluable. However, over the last two decades it has gained considerable attention due to its specific composition in proteins and polar lipids from the milk fat globule membrane (MFGM). The aim of this review is to take stock of current buttermilk knowledge. Firstly, the milk fat globule membrane composition and structure are described. Secondly, buttermilk and its associated products are defined according to the milk fat making process. Structure and mean composition of these products are summarized from recent dairy research data and related to technological properties, especially the emulsifying properties provided by MFGM components. Finally, new applications are presented, leading to promising valorizations of buttermilk and its derivate products.
1. Introduction
1Buttermilk is the term used to refer to the liquid phase released during churning (destabilization) of cream in the butter making process (Morin et al., 2007a). For many years, buttermilk has been considered as the invaluable by-product of the milk fat industry. The worldwide production of buttermilk could be considered close to that of butter production (Morin et al., 2007a), which was estimated at around 8.6 x 106 t in 2006 (FAOSTAT, 2006). Fresh buttermilk consumption remains marginal in Europe. To counter the increasing solids disposed as waste, buttermilk is used in animal feed or dried to be incorporated in dairy or bakery products as an emulsifying agent.
2Over the last two decades, many studies reviewed in this paper have revealed new valorization potentialities for buttermilk, specifically as a source of high added-value components. Like skimmed milk and whey, buttermilk contains lactose, minerals, caseins, and serum proteins which can be extracted, purified and valorized in very distinct food or non food applications. Additionally, buttermilk has attracted considerable interest due to its high content of residual milk fat globule membrane (MFGM). This biological membrane ensures structural integrity, protection and stability of the milk fat in the aqueous phase (Danthine et al., 2000; Ye et al., 2002). MFGM is released into the aqueous phase during cream churning (destabilization of milk fat globules). It contains specific proteins and unique polar lipids (PL) closely associated into a complex structure. Some of these MFGM components are considered beneficial for their various health-related properties (Spitsberg, 2005; Dewettinck et al., 2008). Given this rich composition, buttermilk, whey buttermilk and other by-products derived from the milk fat industry, such as butter-serum, could open a wide range of new means of valorization as emulsifiers, stabilizers, and health promoters in food or non-food outlets.
2. Composition and structure of the milk fat globule membrane
3It has been estimated that the mass of the membrane of fat globules accounts for 2-6% of the total mass of fat globules (Singh, 2006). As MFGM is a biological membrane it is mainly composed of proteins, phospholipids, glycoproteins, neutral lipids, enzymes and other minor components (Danthine et al., 2000). The composition of the MFGM can vary widely depending on many factors such as fat content, fat globule size, diet, breed, health and stage of lactation of the cows (Singh, 2006).
2.1. Lipid composition of the milk fat globule membrane
4Neutral lipids account for a range between 56-80% of total lipids in the MFGM. Triglycerides are the major fraction of neutral lipids (37-68% of total lipids). A major part of these triglycerides appear to originate from contamination by the lipid core during isolation of the MFGM. The other neutral lipids are: diglycerides (9%), monoglycerides (0.7%), esters (0.1-0.8%) and cholesterol (0.2-6.1%) (% of total lipids) (Danthine et al., 2000).
5Polar lipids are composed of phospholipids and sphingolipids; they account for 15-43% of total lipids in the MFGM (Danthine et al., 2000). Among phospholipids, phosphatidylcholine (PC), and phosphatidylethanolamine (PE), are zwitterionic phospholipids that are present in large quantities in the MFGM isolate (35 and 30% respectively). In contrast, anionic phospholipids, phosphatidylinositol (PI) and phosphatidylserine (PS) are present in lower amounts (5 and 3% respectively) (Dewettinck et al., 2008). Three sphingolipids are also found: sphingomyeline (SM) (22%), lactosylceramide (traces) and glucosylceramide (traces). Free fatty acids and gangliosides are also found in the MFGM in minor amounts.
6In a recent work by Fauquant et al. (2007), using a gas chromatography coupled with a mass spectrometry approach, many minor bioactive sterols were detected in the MFGM, e.g. lanosterol, lathosterol, desmosterol, stigmasterol and β-sitosterol.
2.2. Protein composition of the milk fat globule membrane
7MFGM proteins account only for 1-4% of total milk protein. Despite their low level of abundance, MFGM proteins play an important role in various cellular processes and defense mechanisms in the newborn (Cavaletto et al., 2008). Depending on the source, the MFGM is composed of 25-60% of proteins (Danthine et al., 2000; Singh, 2006).
8In the past, identification and characterization of MFGM proteins were mainly based on comparison of electrophoretic mobilities, different staining of the gels, molecular cloning techniques, reaction with specific antibodies, and identification by N-terminal amino acid sequencing. By these means, major bands were separated and identified: Mucin 1, Xanthine dehydrogenase/oxidase, Mucin 15, Cluster of Differentiation 36, butyrophilin, adipophilin, lactadherin, and fatty acid binding protein (Mather, 2000). Later, proteomic approaches, including mass spectrometric (MS) analysis, provided rapid, unambiguous information on protein identity and further identification of minor proteins. By means of a proteomic approach, human MFGM proteins have been identified (Quaranta et al., 2001; Cavaletto et al., 2002; Charlwood et al., 2002; Fortunato et al., 2003). More recently, four works were published concerning the identification of further bovine MFGM proteins by mass spectrometric (MS) analysis: one-dimensional gel electrophoresis followed by capillary liquid chromatography (LC)-nanospray- tandem mass spectrometry (MS/MS) using a quadrupole time-of-flight (Reinhardt et al., 2006), two-dimensional gel electrophoresis (2-DE) followed by reversed phase- LC- MS/MS (Fong et al., 2007), direct LC-MS/MS and 2-DE followed by matrix-assisted laser desorption/ionization time-of-flight MS (Smolenski et al., 2007), and 2-DE followed by matrix-assisted laser desorption/ionization tandem-time-of-flight (Vanderghem et al., 2008). Despite great advancement in identification of minor MFGM proteins, little is known about their function in the MFGM.
9According to Keenan et al. (2006), about 28 different enzymes or enzymatic activities have been detected in MFGM preparations of cows' milk, e.g. Xanthine dehydrogenase/oxidase, 5'-Nucleotidase, γ-Glutamyl transpeptidase, Catalase, Plasmin, Aldolase. Some of these enzymes may possibly come from material entrained in cytoplasmic crescents.
2.3. Structure of the milk fat globule membrane
10The structure of the MFGM, inspired from the models of Danthine et al. (2000), Evers (2004), Michalski et al. (2005) and Keenan et al. (2006), is schematically represented in figure 1. The MFGM is composed of a tri-layer structure. First, there is an inner monolayer that probably covers the intracellular lipid droplets and that originates from the endoplasmic reticulum and possibly other intracellular compartments. In this monolayer, the hydrophobic tails of the polar lipids are in contact with the triglyceride-rich core. Secondly, an outer bilayer that originates from the secretory cell apical plasma membrane, where the outermost hydrophilic head groups of the polar lipids are in contact with the aqueous phase of milk. This bilayer has an electron-dense coat material on the inner face. Some globules present inclusions called “cytoplasmic crescent” entrained between the globule and the surrounding membrane (Danthine et al., 2000; Keenan et al., 2006).
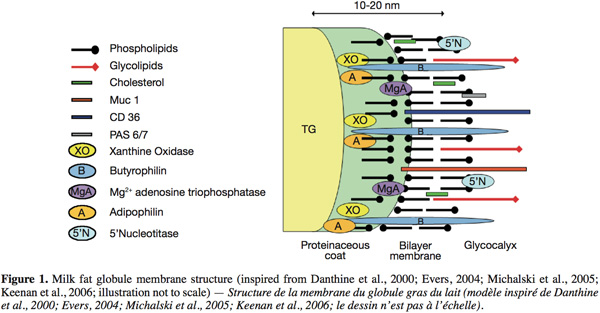
11Concerning the lipids, Deeth (1997) suggested that the polar lipids in the MFGM have an asymmetric distribution like other biological membranes. The outside of the membrane is mainly composed of PC and SM, and the inner surface is mainly composed of PE, PS and PI.
12Regarding the MFGM proteins, a number of techniques have been employed in the past in order to elucidate their disposition. These techniques comprise: morphological studies by means of electron microscopy; surface studies such as labeling techniques, use of lectins, enzyme attack, dissociating agents and detergents (McPherson et al., 1983). In these techniques, identification of the labeled surface proteins, peripheral proteins released after utilization of dissociating agents, or proteins remaining after a proteolytic attack, was based principally on comparison of their relative electrophoretic mobilities in one dimension sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
13Even if much knowledge has been gained about the disposition of the MFGM components, questions remain and the model needs to be detailed.
3. Definition, types and production of buttermilk and butter serum
14Buttermilk is defined by the majority of authors as the aqueous phase released during churning of cream in the butter manufacturing (Corredig et al., 1997; Sodini et al., 2006; Morin et al., 2007a). This global definition includes a wide range of milk fat by-products with various compositions according to the raw material used, pre-treatment conditions and butter making process.
15The production of dairy cream, butter and anhydrous milk fat (AMF) concentrates the lipid phase of milk and releases the serum aqueous phase leading to skimmed milk, buttermilk and butter serum respectively. Each of these by-products has its specific composition. The cream, butter, AMF and by-products (buttermilk, whey buttermilk and butter serum) obtention process are presented in figure 2 and described in this section.
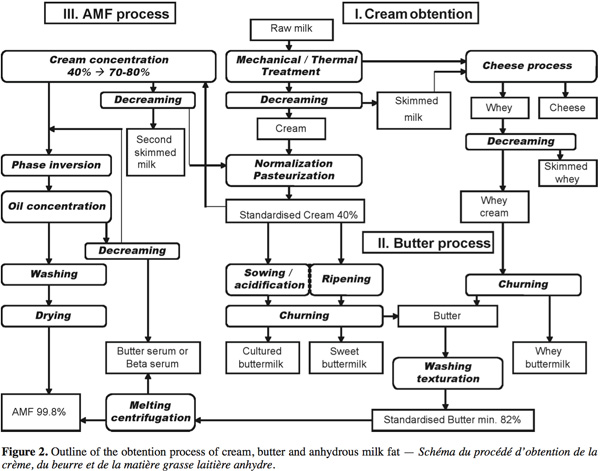
3.1. Cream obtention
16After harvest, raw milk is cold stored and decreamed by centrifugation to adjust the fat content in skimmed milk and concentrate milk fat globules into cream. This cream is generally normalized at around 40% fat and pasteurized. The whey produced by cheese industries is weakened in casein but contains residual milk fat globules. A whey cream is obtained by decreaming of whey and it can be churned leading to a whey buttermilk.
3.2. Butter process
17The cream-to-butter transformation is a complex phenomenon during which the oil-in-water emulsion is reversed into a water-in-oil emulsion leading to aqueous phase separation. The complete explanation of the butter process has been broadly detailed in Mulder et al. (1974), Evers (2004) and Funahashi et al. (2008). The butter process consists of 3 steps: ripening, churning and working with washing.
18Ripening. Ripening of pasteurized cream is a first crucial step which influences both the fat globule destabilization and sensorial quality of butter. Depending on ripening conditions including temperature, time and eventually added ferments, physical changes and biochemical reactions induce different modifications. Physical treatment aims to manage the milk fat crystallization in order to reduce lipid loses in buttermilk during churning and to shape the butter texture. The biological ripening consists of sowing pasteurized cream with specific lactic ferments (starters, bacteria as Lactococcus lactis subsp. cremonis, Lactococcus lactis biovar.). These cultured cream starters lower acidity through lactic acid production and develop typical butyric aromas.
19Consequently, various types of buttermilk can be produced from ripening milk fat. In the manufacture of European style butter, the churning of cultured cream produces cultured buttermilk. Commercial buttermilk is sweet buttermilk, the by-product of churning sweet cream into butter (Sodini et al., 2006).
20Churning. Destabilization of milk fat globules occurs during mechanical agitation of cream in the presence of air, leading to disruption of the thin MFGM surrounding and stabilizing fat globules. Released fat aggregates into a solid lipid matrix forming butter. Discarded residual MFGM fragments are recovered into the aqueous phase with most of the proteins, lactose and minerals contained in the cream (Corredig et al., 1997; Morin et al., 2006). Following this same separation mechanism known since Antiquity, the technological process of butter manufacturing has progressed from discontinuous agitation in farm churns to different continuous churning processes developed by the dairy fat industry. The phase inversion in the butter-making process is widely dependent upon ripening cream properties such as cream acidity, fat content, lipid crystallization, MFGM stability and controlled technological parameters of temperature and shear rate on the beater. The effects of pH, fat globule size, fat content, churning time, and temperature profile and rotation rate have been studied by Mulder et al. (1974) in traditional butter making. More recently, Funahashi et al. (2008) modeled the influence of churning characteristics in continuous butter manufacturing on the water content of the butter.
21Working and washing. The churning of 40% milk fat cream presents an average butter yield of 50%, leading to a roughly equal production of buttermilk and butter (Morin et al., 2007a). The Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO) have normalized the water content of butter at a maximum of 16% (Funahashi et al., 2008). Consequently, the butter resulting from churning of fat globules is washed with water, adjusted for salt, acidity and desirable aromas, before being mechanically textured into a butter making machine, butyrificator and chiller.
3.3. Anhydrous milk fat process
22The anhydrous milk fat (AMF) process is an innovative way that the dairy lipid industry has developed to produce new fat products with specific technofunctional properties. Based on the milk fat fractionation, the process leads to the separation of AMF and butter serum, the aqueous phase of butter. A first way consists of melting standardized butter (at 50°C) and centrifuging it to enhance the separation of butter oil and serum (process used in the studies of Rombaut et al., 2006 and Britten et al., 2008). A second process described schematically in Pérennou (1999) concentrates standardized cream from 40% to 70-80% before the phase inversion and the oil concentration leading to the AMF. The buttermilk resulting from this process is decreamed; the lipid phase returns to the destabilization process and the aqueous phase is called butter serum or beta serum.
4. Composition of buttermilk and butter serum
23Many recent studies on buttermilk aim to assess the compositional and functional properties of different buttermilks (Wong et al., 2003a; Govindasamy-Lucey et al., 2006; Sodini et al., 2006) and butter serum (Britten et al., 2008) or to evaluate the potentialities to concentrate certain high-value components from the buttermilk (Corredig et al., 1997; Morin et al., 2006; 2007b; Rombaut et al., 2007a; 2007b). The composition of dry matter, lactose, proteins, total lipids, polar lipids and ash in different types of buttermilk and butter serums presented in these works are synthesized in table 1 and compared with the composition of raw and skim milk.
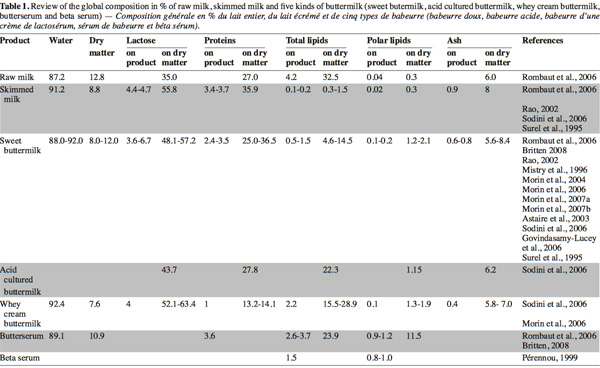
24Buttermilk contains lactose, minerals and skimmed milk proteins (caseins and whey proteins) in the same proportion as skimmed milk (Corredig et al.,1997). However, the concentration of polar lipids on a dry basis in buttermilk has been reported to be four, five and ten times higher than that in skimmed milk, whole milk and cream, respectively (Christie et al., 1987; Rombaut et al., 2005). The majority of polar lipids in milk are originated from the MFGM which is released during churning in the aqueous phase (Malin et al., 1994). According to Morin et al. (2007a), MFGM could represent less than 5% of total solids in buttermilk.
25Except for total lipids and polar lipids, the protein content of sweet and cultured buttermilk is comparable with that of skimmed milk. Nevertheless, certain authors note a slightly lower protein concentration in sweet and cultured buttermilk than for skimmed milk (25-36.5% compared to 36%) (Surel et al., 1995; Scott et al., 2003b; Sodini et al., 2006). Despite washing butter, a small quantity of proteins from sweet or cultured cream is lost in the butter. Whey buttermilk presents the lowest protein content (13.2-14.1%) due to the coagulation of caseins during cheese making, during which a whey poorer in proteins is released (Sodini et al., 2006; Morin et al., 2006).
26According to Britten et al. (2008), the proportion of proteins in sweet buttermilk is approximately 59% caseins, 23% serum proteins, mainly α-lactalbumin and β-lactoglobulin, and around 19% MFGM proteins. In addition, caseins are extracted in large quantity from whey buttermilk, MFGM and serum proteins constitute the larger part of this buttermilk (Sodini et al., 2006).
27Fat content in buttermilk is related to butter manufacturing conditions and the effectiveness of the fat removal process during the post-treatment of buttermilk. This explains the variation of fat content observed among sweet, cultured and whey buttermilks (Elling et al., 1996; Sodini et al., 2006). Wee et al. (2003) studied the inconsistency of composition in buttermilk powder, which can lead to inconsistency of final product quality. They suggest that the different composition of buttermilk powder in the market could be due to the manufacturing method and the source of milk. Fat content varies from 4.6 to 14.5% in dry matter for sweet buttermilk and can reach nearly 29% in whey buttermilk.
28In recent years, much attention has been paid to the polar lipids from the MGFM, which have become the most widely studied components in recent papers about buttermilk. Polar lipid content in raw milk wavers between 100 and 400 mg.kg-1 as a function of the fat content, season, stage of lactation and feeding conditions of the cows (Christie et al., 1987; Bitman et al., 1990). During the butter making process, the total polar lipids initially present in raw milk are distributed among skimmed milk (52%), buttermilk (22%) and butter (26%) according to Britten et al. (2008) in agreement with Christie et al. (1987) and Rombaut et al. (2005). Phospholipids extracted from skimmed milk during decreaming come from membranous material and very small fat droplets initially present in raw milk (Anderson et al., 1975) as well as from MFGM fragments detached from fat globules during the centrifugal process (Walstra et al., 1982). It is believed that the migration of lipids from MFGM to the serum only occurs in the presence of serum components (Houlihan et al., 1992). The polar lipid content in different types of buttermilk ranges from 1.2 to 2.1% in dry matter. Pérennou (1999), confirmed by recent results of Britten et al. (2008), showed that butter serum contains a higher proportion of phospholipids (up to 1 g.100 g-1 of fresh product). Rombaut et al. (2005) obtained a phospholipid content of 11.5% (w/w) in dry matter in a butter serum and recommend this by-product as an interesting source of polar lipids.
29No significant difference was observed between skimmed milk and sweet buttermilk in relation with the proportion of polar lipids. Nevertheless, Rombaut et al. (2005) and Britten et al. (2008) noted an enrichment of sphingomyeline in butter serum at the expense of phosphatidylethanolamine. The composition of polar lipids obtained in sweet buttermilk is PE 39.0%, PC 24.4%, SM 19.3%, PS 8.3%, and PI 8.9%, whereas, for butter serum the polar lipids distribution is PE 29.2%, PC 24.9%, SM 26.8%, PS 10.1%, PI 9.1% and PS 8.3% (Britten et al., 2008).
5. Buttermilks and milk fat globule membrane fractions: functional properties
30The principal function of MFGM in milk is to stabilize the lipid droplets in the plasma. This function is valued by the utilization of buttermilk as a natural ingredient in food process. The aim of this section is to review the studies concerning the functional properties of MFGM fractions and buttermilk.
5.1. Emulsifying properties of milk fat globule membrane fractions and buttermilk
31Any kind of buttermilk, whey buttermilk or butterserum is found to contain two main types of emulsifying agents: proteins like caseins, whey proteins and MFGM proteins; and lipids like polar lipids derived from the MFGM.
32The surface active properties of milk proteins, which have been largely described in the works of Shimizu et al. (1983), Dickinson (1989), Dickinson et al. (1989), Horne et al. (1995), and Britten et al. (1991), are briefly presented here.
33Caseins are the major proteins of bovine milk and have been extensively studied separately from other milk constituents. These are known to have a disordered structure due to their high proline content (Britten et al., 1991). In addition, they are flexible proteins and have amphiphilic properties in their primary structures that allow adsorption at the interfaces (Shimizu et al., 1983). In particular, ß-casein, at the instant of foaming or emulsification, is able to reduce tension rapidly at the newly formed interface (Dickinson et al., 1989).
34Concerning whey proteins, these are globular structured proteins. They do not lower the surface tension as quickly as caseins but are able to form a tightly-packed viscoelastic structure at the interfaces, favoring long-term stability (Dickinson et al., 1989).
35With regard to the emulsifying components of the MFGM: membrane proteins and phospholipids could be considered as natural emulsifying agents due to their amphiphilic nature.
36In the following section the fundamental studies concerning the emulsification properties of the MFGM are summarized.
37Fundamental studies on the native milk fat globule membrane. The first role of fat globule membrane is to stabilize the fat in milk. According to Phipps et al. (1982), the interfacial tension of fat globules is inferior to 2 mN.m-1 and that could explain in part its emulsifying properties. Some studies have been focused on the emulsifying and surface properties of raw and pure milk fat globule membrane components and are presented below.
38Among the first studies, Shimizu et al. (1980) studied the role of MFGM proteins and phospholipids in the stabilization of fat globules in milk. When proteins of the MFGM, obtained from a washed cream, were digested by papain, the decrease in the stability of the cream was considerable due to clustering of the fat globules. These results suggest that proteins and glycoproteins in MFGM may play an important role in the stabilization of fat by interrupting clustering of fat globules. In addition, removal of the phospholipid's polar head group by phospholipase C produced a marked decrease in the stability of the cream emulsion related to a coalescence of fat globules and oiling-off. Shimizu et al. (1980) concluded that phospholipids interrupt coalescence of fat globules by the repulsive forces derived from the ionogenic groups of the phospholipids.
39Subsequently, Kanno (1989) demonstrated that MFGM suspension extracted from fresh raw milk could successfully emulsify butter oil and yield stable emulsions. Different concentrations of membrane were tested in order to assess the emulsion stability, the whippability and the foam stability. They found that at 2% MFGM (80 mg of MFGM.g-1 of fat), emulsifying activity and emulsion stability were maximal.
40Using the film balance technique, Innocente et al. (1997) studied the surface and mechanical properties of a soluble fraction of MFGM obtained from raw cream. The experiments were performed at different temperatures in order to imitate the conditions of different dairy-food processing operations such as heat treatments, milk cream ripening and mechanical treatments. This study clearly shows that temperature greatly modifies the surface properties of MFGM films. These changes were associated with the percentage of crystalline triglycerides and the phospholipids transition (Innocente et al., 1997; Danthine et al., 2000; Laadhar Karray et al., 2006).
41Finally, Corredig et al. (1998c) characterized the interface of emulsions prepared with a fraction of MFGM isolated from fresh raw cream. Proteins and phospholipids from the MFGM were adsorbed at the interface forming a new membrane that was affected very little by the presence of surfactants such as Tweens and Triton-X and proteins such as caseins and β-lactoglobulin added to the emulsion. This behavior is very different from that of emulsions stabilized by other milk proteins. They concluded that the phospholipids present in the MFGM material adsorbed at the interface lowered the interfacial tension, causing a greater resistance to further exchange of surfactant.
42Emulsifying properties of industrial buttermilk and isolates. The utilization of pure MFGM, extracted from raw cream, as a functional ingredient is not possible from an economic point of view (Danthine et al., 2000). However, the MFGM properties could be valued in the by-product of butter manufacture, buttermilk. There is an increasing interest in isolating the MFGM and/or specific fractions from dairy products and by-products (Dalemans et al., 2008). The emulsifying properties of buttermilks and isolates are discussed in the following section.
43According to Elling et al. (1996) and Scott et al. (2003a) MFGM fragments reassociate to the interfacial layer during the recombination of cream with sweet buttermilk and butter derived aqueous phase. Similar findings were reported by Corredig et al. (1998a). They prepared emulsions with commercial buttermilk and soybean oil and analyzed the surface coverage of protein adsorbed at the interface by SDS-PAGE. They found that caseins, MFGM proteins and whey proteins were present. Caseins made up about 50% of the total protein adsorbed. Elling et al. (1996) stated that the amount of phospholipids incorporated in the reconstituted membrane could vary if the homogenization pressure is controlled.
44Functional properties of commercial buttermilk solids have been compared to skimmed milk and whey in oil-in-water emulsions. Wong et al. (2003a) reported that commercial buttermilk showed limited functional properties beyond emulsification capacity and stability over those observed for skimmed milk. In contrast, Scott et al. (2003a) demonstrated that buttermilk emulsions remained stable for a longer time during storage compared to creams formulated with skimmed milk. Their findings were corroborated by other investigators (Sodini et al., 2006) who found that different kinds of buttermilk (commercial sweet, sour and whey buttermilk) showed higher emulsification properties compared to skimmed milk and whey.
45Several studies have focused on obtaining concentrated MFGM isolates from an industrial source of buttermilk. Corredig et al. (1997) isolated membrane-derived material from an industrial source of buttermilk to investigate the potential utilization of the by-product in oil-in-water emulsions. The MFGM isolates were obtained from commercial buttermilk by ultracentrifugation following the addition of sodium citrate to dissociate the casein micelles. This MFGM isolate proved to have very poor emulsifying properties compared to the whole buttermilk isolate. The authors concluded that heat treatment of cream at source and churning causes a high degree of aggregation and changes in the functional properties of membrane proteins and phospholipids. A similar work was undertaken by Roesch et al. (2004) when the differences in functionality between MFGM isolates and a buttermilk concentrate were tested. Their studies demonstrated that MFGM isolates, obtained from representative highly heat-treated buttermilk, showed better creaming stability and smaller oil droplet size distribution than whole buttermilk concentrate samples. These findings were different from the previous results on MFGM isolates. It should be emphasized that the variability and quality of cream, pre-treatment of cream during processing of butter and the method to obtain concentrated MFGM isolates could influence the final functionality. The same group (Corredig et al., 1998b) investigated the changes occurring in the MFGM when cream was heated before butter-making. Heat treatment of the cream caused whey proteins to associate with the MFGM, affecting the solubility and diminishing the amount of iron and the emulsifying properties of the MFGM isolates. Even temperatures as low as 65°C strongly affected the functional properties of the membrane fraction. Recent work conducted by Gassi et al. (2008) shows that the heat treatment of cream affects the physicochemical properties of sweet buttermilk. The heat treatment induced a significant decrease in soluble protein content in buttermilks as well as an increase in the buttermilk phospholipid/fat ratio.
46Studies on the functionality of buttermilk are promising. However, more research is needed in this field. The emulsifying properties of butter serum can be determined and compared to that of buttermilk. Moreover, no work has been focused on the stability of reconstituted buttermilk emulsion and the resistance of the membrane towards coalescence. Nevertheless, the most typical defect related to recombined emulsions is the phase separation and coalescence of droplets during quiescent storage (Dalgleish, 2004). Optimal formulation and high quality membrane material are fundamental for the prevention of coalescence for long shelf-life products.
5.2. Applications of buttermilk and milk fat globule fractions in food: physico-chemical, technofunctional and sensory properties
47The economic and ecological valorization of a by-product such as buttermilk is obvious. To our knowledge, at present the industrial applications of buttermilk are limited to the addition of this by-product in recombined cream and bread. However, the scientific literature pointed out new properties and suggested the utilizations of buttermilk and MFGM isolates in a range of other foodstuffs. In this section, scientific works regarding the applications and properties of MFGM fractions and buttermilk in food are described.
48The applications are mainly oriented to the dairy sector in products such as recombined cream, recombined evaporated milk, yogurt, cheddar cheese, mozzarella, pizza cheese and low fat cheese. Other non-dairy product applications are bread and natural juice. The modified properties in the final products include emulsification, stability, moisture retention, yield, heat stability, texture and flavor (Table 2).
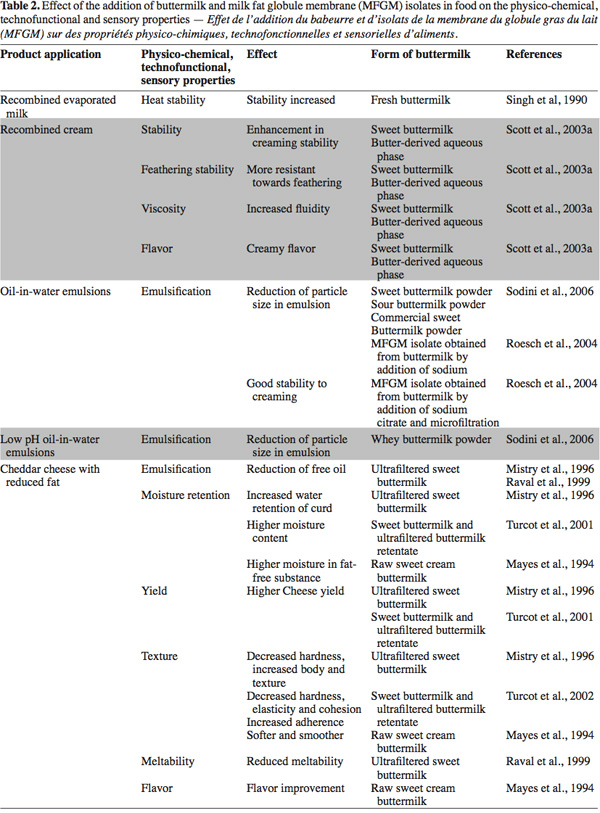
49Singh et al. (1990)'s first studies in the dairy sector evaluated the effect on heat stability of recombined evaporated milk by adding 10% (v/v) of fresh buttermilk to skimmed milk. The authors showed that overall stability was increased in milk collected during the period from October to March, but the extent of stabilization varied. The composition of the newly formed fat globule membranes is a major factor in determining heat stability. The authors hypothesized that surface-active agents in buttermilk (phospholipids and lipo-proteins) displaced skimmed milk protein from the membrane during homogenization. The authors stated that further research is needed on the effects of homogenization and the adsorption of proteins onto fat globules in relation to heat stability of recombined evaporated milk.
50In dairy emulsions, based on their emulsifying properties, the use of buttermilk and MFGM isolate in recombined oil-in-water emulsions considerably reduced the particle size (Roesch et al., 2004; Sodini et al., 2006) and enhanced creaming stability (Scott et al., 2003a; Roesch et al., 2004).
51Concerning cheeses, in an earlier study, Law et al. (1973) investigated the influence of the MFGM addition (by varying the concentration of buttermilk addition) on flavor and lipolysis of cheddar cheese. Their study had demonstrated that the flavor of cheddar cheese is not extensively affected by the concentration of phospholipids in the formulation. They concluded that development of the typical flavor of cheddar cheese is mainly due to triglycerides. However, MFGM material could prevent an undesired rancid flavor, as it adsorbs at the interface, protecting the lipid core from excessive lipolysis. In contrast, in the review of Joshi et al. (1994), during the ripening stage, the proteolysis and the lipolysis increased in the different types of cheese containing buttermilk solids compared to the control sample. These findings were corroborated by the research of Mayes et al. (1994). In their study they found that the addition of raw buttermilk homogenized with cream for the manufacturing of low-fat cheddar cheese slightly improved the textural and flavor characteristics of cheese. They hypothesized that buttermilk enzymes could act on the lipid substrate. More evidence is presented by Samples (1985), where he found that active n-glutamyl transpeptidase was related to two major volatile compounds in cheddar cheese. He pointed out that MFGM is a source of this enzyme.
52Later, the inclusion of 5% of concentrated ultra filtered sweet buttermilk in different types of low-fat cheddar or mozzarella cheese, reduced free-oil formation (Mistry et al., 1996; Poduval et al., 1999; Raval et al., 1999). The emulsification of fat in such cheese types could be improved by the phospholipids contained in buttermilk (Raval et al., 1999).
53Another interesting property observed in cheeses enriched with buttermilk was an increased cheese yield (Mistry et al., 1996; Turcot et al., 2001; Govindasamy-Lucey et al., 2006) due to the water retention capacity of phospholipids and denatured whey proteins (Turcot et al., 2001). However, high water retention could impart negative effects in cheese such an increase in the acidity during the ripening period (Joshi et al., 1994), reduction of the shelf life and modifications in the texture.
54A recent study, conducted by Morin et al. (2008), shows that heat treatment of the cream is an important reason for the buttermilk coagulation problems in cheeses. Pasteurization of cream modifies the surface of MFGM particles, and this may explain why buttermilk has poor coagulation properties and therefore yields rennet gels with texture defects.
55Ultrafiltered buttermilk could be used as a suitable ingredient to manipulate the degree of melting (Poduval et al., 1999). In reduced-fat cheddar cheese, mozzarella cheese and pizza cheese, addition of buttermilk reduced meltability (Poduval et al., 1999; Raval et al., 1999; Govindasamy-Lucey et al., 2006). Poduval et al. (1999) and Raval et al. (1999) hypothesized that the components of the MFGM formed an integral part of the protein matrix and caused more extensive cross links, resulting in a structure that did not melt well.
56Furthermore, the addition of buttermilk decreased hardness, increased body and improved the texture of cheeses (Mayes et al., 1994; Mistry et al., 1996; Poduval et al., 1999; Turcot et al., 2002). Similarly, the addition of up to 4.8% sweet buttermilk powder to low fat yogurt enhanced its sensory qualities by softening the product and making it smoother (Trachoo et al., 1998).
57Recently evidence was presented that buttermilk solids cause antioxidant activity by acting as a reducing agent to scavenge peroxide and hydroxyl radical and sequester both Fe+2 and Fe+3 (Wong et al., 2003b). The authors recommend the utilization of buttermilk in foods in order to prevent lipid peroxidation.
58Buttermilk is also used in baked goods because of its typical flavor (Malin et al., 1994; Bilgin et al., 2006) and also has a retarding effect on crumb firmness (Bilgin et al., 2006).
59Another innovative utilization of buttermilk is the supplementation of natural fruit beverages with this by-product (Farah et al., 1981; Shukla et al., 2004). These fruit beverages combine the high protein and mineral contents of buttermilk with the vitamins of the fruit in a cheaper product.
60In conclusion, different types of buttermilk and MFGM isolates have been proven to be successful in improving some food characteristics. An example is the effect of buttermilk supplementation on reduced fat cheeses, as it gives products characteristics that are similar to or better than those of their full fat counterparts. However, the quantities of buttermilk used as a food ingredient must be carefully measured so as to avoid affecting the functional and sensory properties of final products. High concentrations could negatively affect their properties and result in the development of off-flavors.
5.3. Other applications of the milk fat globule membrane: pharmaceutical applications and formation of liposomes
61Conventional phospholipids could be obtained from natural origins such as soybean, egg yolk or synthesized by various fatty acid compositions. As for the other sources, the phospholipid fractions from the MFGM could be used for emulsions or liposome formulations for pharmaceutical or cosmetic applications. Moreover, the special, unique composition of the MFGM phospholipids fraction (50% saturated, 35% monounsaturated and 15% poly-unsaturated fatty acids) makes products resistant to oxidation.
62Due to their natural origin, MFGM components may be safe for oral administration, and they are an interesting alternative to synthetic emulsifiers. MFGM has been tested as a novel emulsifier to enhance intestinal drug absorption of vitamin D3 (Liu et al., 1991), epidermal growth factor (Adachi et al., 1993), α-linolenic acid (Yuasa et al., 1994) and cyclosporine (Sato et al., 1994).
63The MFGM contains a type of sphingolipid found in animal cell membranes: the SM. As presented in point 2.1., among sphingolipids, SM is present in large quantities. Malmsten et al. (1994) studied the properties of SM extracted from bovine milk. They found that the physicochemical properties of SM from bovine milk are similar to those of satured PC. They demonstrated that it was possible to form liposomes readily in the presence of cholesterol. SM from bovine milk could be used for liposome dispersions in order to be used as drug delivery systems and in the preparation of emulsions for dermal applications and cosmetics.
64Later, Miura et al. (2004) tested the emulsifying properties of bovine milk phospholipids by reconstituting cream using butter oil. They found that phospholipids extracted from bovine milk dispersed in the oil phase stabilize the oil-in-water emulsion. Additionally, the major polar lipids from bovine milk (PC, PE and SM) were studied separately for their emulsifying properties. They reported that PC dispersed in butter oil was able to prevent the solidification of the emulsion, whereas PE and SM had no such effects. The author hypothesized that the structural differences of the phospholipids may give rise to the different abilities of phospholipids to stabilize emulsions.
65Recently, Thompson et al. (2006b) produced liposomes from a phospholipid-rich MFGM fraction, using a microfluidizer. With this technique, a large volume of liposomes can be produced in a continuous and reproducible manner, avoiding the use of detergents, solvents or alcohol. Milk fat globule membrane liposomes are more stable than their soya counterparts in a range of pH conditions, at a variety of storage and processing temperatures, and in the presence of mono- and divalent cations (Thompson et al., 2006a).
6. Conclusion
66This literature review revised the recent advances in composition and structure of the MFGM, butter serums and buttermilks, and described the functional properties of these products.
67By means of new methods, great advancement in identification of minor components of the MFGM has been reached. However, little is known about their function in the MFGM structure and their biological function. In parallel, much knowledge has been gained about the disposition of the MFGM components but the structure is not known in detail and works done on the subject are not always coincident.
68Although buttermilk has been considered as an invaluable by-product of the milk fat industry, recently it has attracted considerable interest due to its high MFGM content.
69Studies on the functionality of MFGM fractions and buttermilk are promising; they have been proven to be successful in improving some food characteristics. However, more research is needed in this field (e.g. studies on the interfacial properties of MFGM and buttermilk; assessment of the stability of reconstituted buttermilk emulsion and the resistance of the membrane towards coalescence; effect of heat treatment of cream at source in MFGM fractions or buttermilk, in the functional properties of final products). In addition, other by-products derived from the milk fat industry, such as butter serum, remain to be explored for their functional properties.
70MFGM fractions and buttermilk offer many other possibilities that can be exploited by food technologists. The polar lipid fraction of the MFGM, in addition to having good emulsification properties in food formulation, could be used safely for pharmaceutical preparation of emulsions for oral use and liposomal formulations. Another way of further enhancing these by-products is to obtain more specific fractions and to find wider applicability and high-value applications.
71In conclusion, the MFGM topic has been studied for long time and continues to interest the scientists and technologists. The knowledge about the MFGM and buttermilk has increased in the last years due to the use of more sophisticated analytical techniques. In parallel, the studies on isolation/cracking of MFGM from industrial sources and the number of applications of the MFGM and its fractions have considerably increased as denoted by the research literature.
72Abbreviations
732-DE: two-dimensional gel electrophoresis
74AMF: anhydrous milk fat
75LC: liquid chromatography
76MALDI-TOF: matrix-assisted laser desorption/ionization time-of-flight
77MFGM: milk fat globule membrane
78MS: mass spectrometry
79MS/MS: tandem mass spectrometry
80PC: phosphatidylcholine
81PE: phosphatidylethanolamine
82PI: phosphatidylinositol
83PL: polar lipids
84PS: phosphatidylserine
85SM: sphingomyelin
86Acknowledgement
87The first author gratefully thanks the University of Liege - Gembloux Agro-Bio Tech for a grant.
Bibliographie
Adachi I. et al., 1993. Possibility of lymphatic absorption of epidermal growth factor from intestine. Yakugaku Zasshi, 113, 256-263.
Anderson M. & Brooker B.E., 1975. Loss of material during the isolation of milk fat globule membrane. J. Dairy Sci., 58, 1442-1448.
Astaire J.C., Ward R., German J.B. & Jiménez-Flores R., 2003. Concentration of polar MFGM lipids from buttermilk by microfiltration and supercritical fluid extraction. J. Dairy Sci., 86, 2297-2307.
Bilgin B., Daglioglu O. & Konyali M., 2006. Functionality of bread made with pasteurized whey and/or buttermilk. Ital. J. Food Sci., 18, 277-286.
Bitman J. & Wood D.L., 1990. Changes in milk fat phospholipids during lactation. J. Dairy Sci., 73, 1208-1216.
Britten M. & Giroux H.J., 1991. Emulsifying properties of whey protein and casein composite blends. J. Dairy Sci., 74, 3318-3325.
Britten M., Lamothe S. & Robitaille G., 2008. Effect of cream treatment on phospholipids and protein recovery in butter-making process. Int. J. Food Sci. Technol., 43, 651-657.
Cavaletto M. et al., 2002. A proteomic approach to evaluate the butyrophilin gene family expression in human milk fat globule membrane. Proteomics, 2, 850-856.
Cavaletto M., Giuffrida M.G. & Conti A., 2008. Milk fat globule membrane components: a proteomic approach. Adv. Exp. Med. Biol., 606, 129-141.
Charlwood J. et al., 2002. Use of proteomic methodology for the characterization of human milk fat globular membrane proteins. Anal. Biochem., 301, 314-324.
Christie W.W., Noble R.C. & Davies G., 1987. Phospholipids in milk and dairy products. J. Soc. Dairy Technol., 40, 10-12.
Corredig M. & Dalgleish D.G., 1997. Isolates from industrial buttermilk: emulsifying properties of materials derived from the milk fat globule membrane. J. Agric. Food Chem., 45, 4595-4600.
Corredig M. & Dalgleish D.G., 1998a. Buttermilk properties in emulsions with soybean oil as affected by fat globule membrane-derived proteins. J. Food Sci., 63, 476-480.
Corredig M. & Dalgleish D.G., 1998b. Effect of heating of cream on the properties of milk fat globule membrane isolates. J. Agric. Food Chem., 46, 2533-2540.
Corredig M. & Dalgleish D.G., 1998c. Characterization of the interface of an oil-in-water emulsion stabilized by milk fat globule membrane material. J. Dairy Res., 65, 465-477.
Dalemans D. et al., 2008. Milk ingredient enriched in polar lipids and uses thereof. Patent WO/2008/009636. PCT/EP2007/057247.
Dalgleish D.G., 2004. Food emulsions: their structures and properties. In: Friberg S.E., Larsson K. & Sjöblom J., eds. Food emulsions. 4th ed. New York, NY, USA: Marcel Dekker.
Danthine S. et al., 2000. Évolution des connaissances sur la membrane du globule gras du lait : synthèse bibliographique. Lait, 80, 209-222.
Deeth H., 1997. The role of phospholipids in the stability of milk fat globules. Aust. J. Dairy Technol., 52, 44-46.
Dewettinck K. et al., 2008. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J., 18, 436-457.
Dickinson E., 1989. Surface and emulsifying properties of caseins. J. Dairy Res., 56, 471-477.
Dickinson E., Mauffret A., Rolfe S.E. & Woskett C.M., 1989. Adsorption at interfaces in dairy systems. J. Soc. Dairy Technol., 42, 18-22.
Elling J.L. & Duncan S.E., 1996. Physical properties of 20% milk fat reformulated creams manufactured from cholesterol-reduced butteroil. J. Food Sci., 61, 375-378.
Evers J.M., 2004. The milk fat globule membrane-composition and structural changes post secretion by the mammary secretory cell. Int. Dairy J., 14, 661-674.
FAOSTAT, 2006. Subject: 2005 World Butter and Ghee Production, http://faostat.fao.org, (12/06/08).
Farah Z. & Bachman M., 1981. Buttermilk flavoured with natural fruit juice: an example of a product development for developing countries. Lebensm. Wiss. Technol., 14, 276-277.
Fauquant C. et al., 2007. Membrane phospholipids and sterols in microfiltered milk fat globules. Eur. J. Lipid Sci. Technol., 109, 1167 - 1173.
Fong B.Y., Norris C.S. & MacGibbon A.K.H., 2007. Protein and lipid composition of bovine milk-fat-globule membrane. Int. Dairy J., 7, 275-288.
Fortunato D. et al., 2003. Structural proteome of human colostral fat globule membrane proteins. Proteomics, 3, 897-905.
Funahashi H. & Horiuchi J., 2008. Characteristics of the churning process in continuous butter manufacture and modelling using an artificial neutral network. Int. Dairy J., 18, 323-328.
Gassi J.-Y., Famelart M.-H. & Lopez C., 2008. Heat treatment of cream affects the physicochemical properties of sweet buttermilk. Dairy Sci. Technol., 88, 369-385.
Govindasamy-Lucey S. et al., 2006. Influence of condensed sweet cream buttermilk on the manufacture, yield, and functionality of pizza cheese. J. Dairy Sci., 89, 454-467.
Horne D.S. & Leaver J., 1995. Milk proteins on surfaces. Food Hydrocolloids, 9, 91-95.
Houlihan A.V. et al., 1992. Interactions between the bovine milk fat globule membrane and skim milk components on heating whole milk. J. Dairy Res., 59, 187-195.
Innocente N., Blecker C., Deroanne C. & Paquot M., 1997. Langmuir film balance study of the surface properties of a soluble fraction of milk fat-globule membrane. J. Agric. Food Chem., 45, 1559-1563.
Joshi N.S., Thakar P.N. & Jana A.H., 1994. Utilization of butter milk in cheese making: a review. Indian Food Packer, 48, 59-65.
Kanno C., 1989. Emulsifying properties of bovine milk fat globule membrane in milk fat emulsion: conditions for the reconstitution of milk fat globules. J. Food Sci., 54, 1534-1539.
Keenan T.W. & Mather I.H., 2006. Intracellular origin of milk fat globules and the nature of the milk fat globule membrane. In: Fox P.F. & McSweeney P.L.H., eds. Advanced dairy chemistry. New York, NY, USA: Springer.
Laadhar Karray N., Danthine S., Blecker C. & Attia H., 2006. Contribution to the study of camel milk fat globule membrane. Int. J. Food Sci. Nutr., 57, 382-390.
Law B.A., Sharpe M.E., Chapman H.R. & Reiter B., 1973. Relationship of milk fat globule membrane material to flavor development in cheddar cheese. J. Dairy Sci., 56, 716-723
Liu H.X., Adachi I., Horikoshi I. & Ueno M., 1991. Promotion of intestinal drug absorption by milk fat globule membrane. Yakugaku Zasshi, 111, 510-514.
Malin E.L. et al., 1994. Detection of adulteration of buttermilk powder by gel electrophoresis. J. Dairy Sci., 77, 2199-2206.
Malmsten M., Bergenstahl B., Nyberg L. & Odham G., 1994. Sphingomyelin from milk: characterization of liquid crystalline, liposome and emulsion properties. J. Am. Oil Chem. Soc., 71, 1021-1026.
Mather I.H., 2000. A review and proposed nomenclature for major proteins of the milk-fat globule membrane. J. Dairy Sci., 83, 203-247.
Mayes J.J., Urbach G. & Sutherland B.J., 1994. Does addition of buttermilk affect the organoleptic properties of low-fat cheddar cheese? Aust. J. Dairy Technol., 49, 39-41.
McPherson A.V. & Kitchen B.J., 1983. Reviews of the progress of dairy science: the bovine milk fat globule membrane- its formation, composition, structure and behaviour in milk and dairy products. J. Dairy Res., 50, 107-133.
Michalski M.C. et al., 2005. Size distribution of fat globules in human colostrum, breast milk, and infant formula. J. Dairy Sci., 88, 1927-1940.
Mistry V.V., Metzger L.E. & Maubois J.L., 1996. Use of ultrafiltered sweet buttermilk in the manufacture of reduced fat cheddar cheese. J. Dairy Sci., 79, 1137-1145.
Miura S., Tanaka M., Suzuki A. & Sato K., 2004. Application of phospholipids extracted from bovine milk to the reconstitution of cream using butter oil. J. Am. Oil Chem. Soc., 81, 97-100.
Morin P., Jiménez-Flores R. & Pouliot Y., 2004. Effect of temperature and pore size on the fractionation of fresh and reconstituted buttermilk by microfiltration. J. Dairy Sci., 87, 267-273
Morin P., Pouliot Y. & Jiménez-Flores R., 2006. A comparative study of the fractionation of regular buttermilk and whey buttermilk by microfiltration. J. Food Eng., 77, 521-528.
Morin P., Britten M., Jiménez-Flores R. & Pouliot Y., 2007a. Microfiltration of buttermilk and washed cream buttermilk for concentration of milk fat globule membrane components. J. Dairy Sci., 90, 2132-2140.
Morin P., Jiménez-Flores R. & Pouliot Y., 2007b. Effect of processing on the composition and microstructure of buttermilk and its milk fat globule membranes. Int. Dairy J., 17, 1179-1187.
Morin P., Pouliot Y. & Britten M., 2008. Effect of buttermilk made from creams with different heat treatment histories on properties of rennet gels and model cheeses. J. Dairy Sci., 91, 871-882
Mulder H. & Walstra P., 1974. The milk fat globule. Wageningen, The Netherlands: Center for agricultural Publishing and Documentation.
Pérennou H., 1999. Le fractionnement à sec, un transfert de technologie réussi. Rev. Laitière Fr., 595, 22-23.
Phipps L.W. & Temple D.M., 1982. Surface properties of milk fat globules: interfacial tension studies. J. Dairy Res., 49, 61-72.
Poduval V.S. & Mistry V.V., 1999. Manufacture of reduced fat mozzarella cheese using ultrafiltered sweet buttermilk and homogenized cream. J. Dairy Sci., 82, 1-9.
Quaranta S. et al., 2001. Human proteome enhancement: high-recovery method and improved two-dimensional map of colostral fat globule membrane proteins. Electrophoresis, 22, 1810-1818.
Rao H.G.R., 2002. Mechanisms of flux decline during ultrafiltration of dairy products and influence of pH on flux rates of whey and buttermilk. Desalination, 144, 319-324.
Raval D.M. & Mistry V.V., 1999. Application of ultrafiltered sweet buttermilk in the manufacture of reduced fat process cheese. J. Dairy Sci., 82, 2334-2343.
Reinhardt T.A. & Lippolis J.D., 2006. Bovine milk fat globule membrane proteome. J. Dairy Res., 73, 406-416.
Roesch R.R., Rincon A. & Corredig M., 2004. Emulsifying properties of fractions prepared from commercial buttermilk by microfiltration. J. Dairy Sci., 87, 4080-4087.
Rombaut R., van Camp J. & Dewettinck K., 2005. Analysis of phospho- and sphingolipids in dairy products by a new hplc method. J. Dairy Sci., 88, 482-488.
Rombaut R., Van Camp J. & Dewettinck K., 2006. Phospho- and sphingolipid distribution during processing of milk, butter and whey. Int. J. Food Sci. Technol., 41, 435-444.
Rombaut R. & Dewettinck K., 2007a. Thermocalcic aggregation of milk fat globule membrane fragments from acid buttermilk cheese whey. J. Dairy Sci., 90, 2665-2674.
Rombaut R., Dejonckheere V. & Dewettinck K., 2007b. Filtration of milk fat globule membrane fragments from acid buttermilk cheese whey. J. Dairy Sci., 90, 1662-1673.
Samples D.R., 1985. Some factors affecting the production of volatile sulfhydryl compounds in cheddar cheese slurries. PhD thesis: Texas A&M University (USA).
Sato H. et al., 1994. Enhancement of the intestinal absorption of a cyclosporine derivative by milk fat globule membrane. Biol. Pharm. Bull., 17, 1526-1528.
Scott L.L., Duncan S.E., Sumner S.S. & Waterman K.M., 2003 a. Physical properties of cream reformulated with fractionated milk fat and milk-derived components. J. Dairy Sci., 86, 3395-3404.
Scott L.L. et al., 2003b. Influence of emulsifying component composition on creams formulated with fractionated milkfat. J. Agric. Food Chem., 51, 5933-5940.
Shimizu M., Yamauchi K. & Kanno C., 1980. Effect of proteolytic digestion of milk fat globule membrane proteins on stability of the globules. Milchwissenschaft, 35, 9-12.
Shimizu M., Takahashi T., Kaminogawa S. & Yamauchi K., 1983. Adsorption onto an oil surface and emulsifying properties of bovine αs1-casein in relation to its molecular structure. J. Agric. Food Chem., 31, 1214-1218.
Shukla F.C., Sharma A. & Singh B., 2004. Studies on the preparation of fruit beverages using whey and buttermilk. J. Food Sci. Technol., 41, 102-105.
Singh H., 2006. The milk fat globule membrane-A biophysical system for food applications. Curr. Opin. Colloid Interface Sci., 11, 154-163.
Singh H. & Tokley R.P., 1990. Effects of preheat treatments and buttermilk addition on the seasonal variations in the heat stability of recombined evaporated milk and reconstituted concentrated milk. Aust. J. Dairy Technol., 45, 10-16.
Smolenski G. et al., 2007. Characterisation of host defence proteins in milk using a proteomic approach. J. Proteome Res., 6, 207-215.
Sodini I., Morin P., Olabi A. & Jiménez-Flores R., 2006. Compositional and functional properties of buttermilk: a comparison between sweet, sour, and whey buttermilk. J. Dairy Sci., 89, 525-536.
Spitsberg V.L., 2005. Invited review: bovine milk fat globule membrane as a potential nutraceutical. J. Dairy Sci., 88, 2289-2294.
Surel O. & Famelart M.H., 1995. Ability of ceramic membranes to reject lipids of dairy products. Austr. J. Dairy Technol., 50, 36-40.
Thompson A.K., Haisma D. & Singh H., 2006a. Physical stability of liposomes prepared from milk fat globule membrane and soya phospholipids. J. Agric. Food Chem., 54, 6390-6397.
Thompson A.K. & Singh H., 2006b. Preparation of liposomes from milk fat globule membrane phospholipids using a microfluidizer. J. Dairy Sci., 89, 410-419.
Trachoo N. & Mistry V.V., 1998. Application of ultrafiltered sweet buttermilk and sweet buttermilk powder in the manufacture of nonfat and low fat yogurts. J. Dairy Sci., 81, 3163-3171.
Turcot S., Turgeon S.L. & St-Gelais D., 2001. Effet de la concentration en phospholipides de babeurre dans le lait de fromagerie sur la production et la composition de fromages allégés de type cheddar. Lait, 81, 429-442.
Turcot S., St-Gelais D. & Turgeon S.L., 2002. Affinage de fromages allégés de type cheddar fabriqués à partir de laits enrichis en phospholipides. Lait, 82, 209-223.
Vanderghem C. et al., 2008. Proteome analysis of the bovine milk fat globule: enhancement of membrane purification. Int. Dairy J., 18, 885-893.
Walstra P. & Oortwijn H., 1982. The membranes of recombined fat globules. III. Mode of formation. Neth. Milk Dairy J., 36, 103-113.
Wee J.K. & Smith D.E., 2003. Compositional difference of sweet cream buttermilk. In: Poster presentation, IFT Annual Meeting, July 12-16, 2003, Chicago, IL, USA.
Wong P.Y.Y. & Kitts D.D., 2003a. A comparison of the buttermilk solids functional properties to nonfat dried milk, soy protein isolate, dried egg white, and egg yolk powders. J. Dairy Sci., 86, 746-754.
Wong P.Y.Y. & Kitts D.D., 2003b. Chemistry of buttermilk solid antioxidant activity. J. Dairy Sci., 86, 1541-1547.
Ye A., Singh H., Taylor M.W. & Anema S., 2002. Characterization of protein components of natural and heat-treated milk fat globule membranes. Int. Dairy J., 12, 393-402.
Yuasa H., Sekiya M., Ozeki S. & Watanabe J., 1994. Evaluation of milk fat-globule membrane (MFGM) emulsion for oral administration: absorption of alpha-linolenic acid in rats and the effect of emulsion droplet size. Biol. Pharm. Bull., 17, 756-758.
