- Startpagina tijdschrift
- Volume 19 (2015)
- numéro 4
- Bioreactor design and implementation strategies for the cultivation of filamentous fungi and the production of fungal metabolites: from traditional methods to engineered systems
Weergave(s): 40287 (126 ULiège)
Download(s): 5196 (8 ULiège)
Bioreactor design and implementation strategies for the cultivation of filamentous fungi and the production of fungal metabolites: from traditional methods to engineered systems

Nota's van de redactie
Received on November 28, 2013; accepted on July 27, 2015
Résumé
Conception de bioréacteurs et mise en œuvre de stratégies pour la culture de champignons filamenteux et la production de métabolites d’origine fongique : des méthodes traditionnelles aux technologies actuelles
La production de métabolites d’origine fongique et de conidies à l’échelle industrielle requiert un procédé adéquat à cout relativement bas. À cet effet, beaucoup de facteurs sont analysés et la configuration du bioréacteur à utiliser pour le produit retenu occupe une place prépondérante dans l’analyse. Une approche à suivre pour résoudre le problème relatif à la production de métabolites et de conidies par les champignons consiste à maitriser la montée en échelle du procédé de fermentation avec le respect de caractéristiques biologiques de micro-organismes, tels que les champignons filamenteux pour le cas qui concerne notre étude. En effet, cette montée en échelle est considérée comme l’un des principaux points d’achoppement en technologie de fermentation : ceci en raison de la quasi impossibilité de reproduire exactement les mêmes conditions idéales obtenues en utilisant les petits réacteurs de recherche au moment de les transposer à la production à grande échelle. La présente revue vise à faire le point sur la conception de bioréacteurs et leur mise en œuvre pour la culture de champignons filamenteux et la production de métabolites en fonction des différents stades de développement de champignons d’intérêt industriel. Les cultures solide (semi-solide), submergée et en biofilm ont été prises en compte dans les différentes études. Les différents types de bioréacteurs utilisés pour les trois procédés sont aussi décrits du point de vue technologique.
Abstract
The production of fungal metabolites and conidia at an industrial scale requires an adequate yield at relatively low cost. To this end, many factors are examined and the design of the bioreactor to be used for the selected product takes a predominant place in the analysis. One approach to addressing the issue is to integrate the scaling-up procedure according to the biological characteristics of the microorganism considered, i.e. in our case filamentous fungi. Indeed, the scaling-up procedure is considered as one of the major bottlenecks in fermentation technology, mainly due to the near impossibility of reproducing the ideal conditions obtained in small reactors designed for research purposes when transposing them to a much larger production scale. The present review seeks to make the point regarding the bioreactor design and its implementation for cultivation of filamentous fungi and the production of fungal metabolites according to different developmental stages of fungi of industrial interest. Solid-state (semi-solid), submerged, fermentation and biofilm reactors are analyzed. The different bioreactor designs used for these three processes are also described at the technological level.
Inhoudstafel
1. Introduction
1Three fungal genera are widely used for biotechnological applications: Aspergillus sp., Penicillium sp., and to a lesser extent, Trichoderma sp. Filamentous fungi have the capability to produce extremely large quantities of homologous proteins, including several enzymes that can be used as industrial biocatalysts. Several volatile metabolites (MVOC) can also be produced. However, the production of these metabolites relies on the culture mode at both the quantitative and qualitative levels. Indeed, these fungi are able to grow in different forms: from free mycelium and pellet in liquid phase, to pellet and mycelium to conidia at a solid-air interface. Based on this, different bioreactor strategies have been developed in order to promote a given development stage. In this context, three modes of cultivations can be considered:
2– submerged fermentation where two kinds of morphologies are most often depicted, i.e. free mycelium (with different degrees of ramification) and pellets;
3– semi-solid fermentation which is reflected by the development of mycelium on the surface and at the interior of a solid substrate in the absence of free water;
4– biofilm formation, represented by the development of mycelium on an inert support, such as stainless steel, glass or Teflon material integrated in an adapted reactor.
5Among these three strategies, submerged fermentation is the most widely used mode of fermentation. However, the last two strategies lead to the enhancement of the development of aerial hyphae with the production of conidia on conidiophore position of filaments depending on the development stage of the microorganism. They will be able to generate fungal products of biotechnological interest, since the excretion capacity is increased when fungal biomass is attached on a given support. Barrios-Gonzalez (2012) reported that the solid-state fermentation is considered as an alternative culture method that has gained researchers attention over the past 20 years, and credibility among many industrial corporations. The present review is aimed at understanding the influence of the cultural conditions on the developmental stage of the fungi, as well as on the excretion of secondary metabolites of industrial interest, which highlight the orientation for bioreactor design needed. It is focused on bioreactor, fermentation, strategies for secondary metabolites enhancement and some applications cultivation of Trichoderma, Penicillium and Aspergillus. In the conclusion, a new range of biofilm reactors will be proposed for the improvement of the production of fungal metabolites.
2. Traditional methods and engineered systems applied for the cultivation of filamentous fungi in order to produce the metabolites and conidia
2.1. Basic bioreactor design: from submerged fermentation to biofilm reactor
6A bioreactor can be defined as mechanically stirred vessel in which organisms are cultivated in a controlled manner and/or materials are converted or transformed through specific reactions. Indeed, traditional methods for solid state fermentation include tray, drum and packed bed bioreactors with problems regarding the control of different parameters; while, for submerged fermentation they include continuous stirred-bioreactors, continuous flow stirred-tank reactors, plug-flow reactor, and fluidized-bed reactors. For biofilm processes, the material supporting the microorganism can be used in reactors applied for submerged fermentation and adapted for used in order to cultivate immobilized cells or biofilms with respect to the production of value-added molecules (Muffler et al., 2014).
2.2. Factors affecting bioreactor design
7Many factors contribute to the design of a bioreactor. Durand (2003) reported that compared to submerged fermentation, the solid media used in solid-state fermentation contain less water but an important gas phase existed between the particles. This feature is of great importance because of the poor thermal conductivity of air compared to water. Another point is the wide variety of matrices used in solid-state fermentation, which vary in terms of composition, size, mechanical resistance, porosity and water-holding capacity. All these factors can affect the reactor design and the strategy to control the key parameters. Indeed, with submerged fermentation, it can be considered an approximation that all the media are made up essentially of water. In this environment, temperature and pH regulations are trivial and do not pose problems during the scaling-up of a process.
8With submerged fermentation, the difficulty mainly encountered is related to limitations at the level of oxygen transfer capacity, which depends upon the shape and size of the reactor and the agitation/aeration system used. To characterize this transfer, a parameter, KLa (oxygen transfer coefficient), has been defined. It can be considered as a “similarity invariant”, which means that its value expresses the capacity of the equipment to transfer oxygen independent of the volume of the reactor and, as such, constitutes an important parameter used in scale-up studies in submerged fermentation. With solid-state fermentation, besides oxygen transfer, which can be a limiting factor for some designs, the problems are more complex and affect the control of three important parameters, i.e. temperature, pH and water content of the solid medium. There are also other factors affecting the bioreactor design, namely:
9– the morphology of the fungus (the presence or not of a septum in the hyphae) and, related to this, its resistance to mechanical agitation,
10– the necessity or not of having a sterile process.
11Concerning fungal morphology, as reported by Papagianni (2004), filamentous fungi are morphologically complex microorganisms, exhibiting different structural forms throughout their life cycles. The basic vegetative structure of growth consists of tubular filaments known as hyphae that originate from the germination of a single reproductive conidium or a piece of mycelium. As the hyphae continue to grow, they branch frequently and repeatedly to form a mass of hyphal filaments referred to as a mycelium. When grown in submerged culture, these fungi exhibit different morphological forms, ranging from dispersed mycelial filaments to densely interwoven mycelial masses referred to as pellets, whereas conidiation, as the end point of the fungi’s developmental cycle, is rarely achieved during submerged cultivation, partially due to relatively good nutrient availability and partly to the physical nature of the hyphal wall. Different metabolites are produced by these different forms in submerged culture, so the successful production of fungal metabolites requires detailed knowledge of the growth characteristics and physiology of the fungus considered. Not only does the production of different metabolites require different physiological conditions, but each fungal species is also unique in its anatomical, morphological and physiological development. Thus, for fungal fermentation, the precise physiological conditions and correct stage of development must be established to achieve the maximal product formation. In other words, controlling the form of these microorganisms is a real issue that needs great attention in order to make optimal use of their potential production capacities during their development according to the structure of bioreactor designed.
2.3. Solid-state fermentation processes
12Many examples of solid-state fermentation have been reported by researchers, some of which are depicted in a figure presented by Durand (2003) and others where they represented tray bioreactors, rotating drum bioreactors, packed bed bioreactors and others more sophisticated. Regarding examples of submerged and biofilm fermentations, the different developmental stages observed depend on the processes used to obtain the final product desired at the end of the process. Some examples are outlined in figures 1A, 2A and 3A. The figures confirmed that the biomass that can be accumulated on the support was higher in biofilm reactor (Figure 3B) than its equivalent in submerged reactor, pointing out a possible way of bioprocess intensification (Figure 1A). The same conclusion has been outlined by Seye et al. (2014) concerning immobilisation of microorganisms on support used in fungal biofilm reactor for production of conidia using Aspergillus clavatus. The choice of a bioreactor for a given fermentation is based on the production or productivity yields and the regulation of the system required for the filamentous fungi’s growth. Regarding bioreactors for solid-state fermentation at the pilot and industrial levels, a few categories can be distinguished based on the aeration and mixing strategies. These are: forced or unforced aeration; static, pulsed mixed, or continuously mixed.
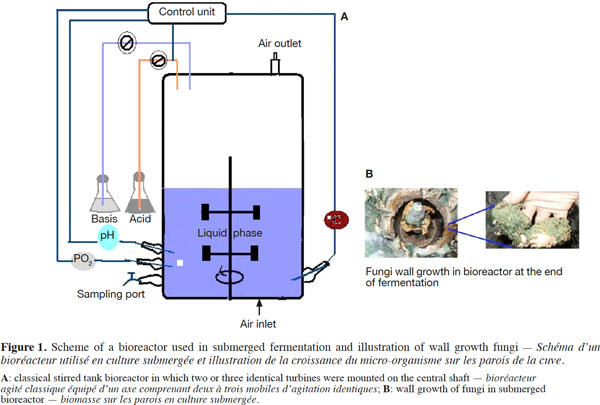
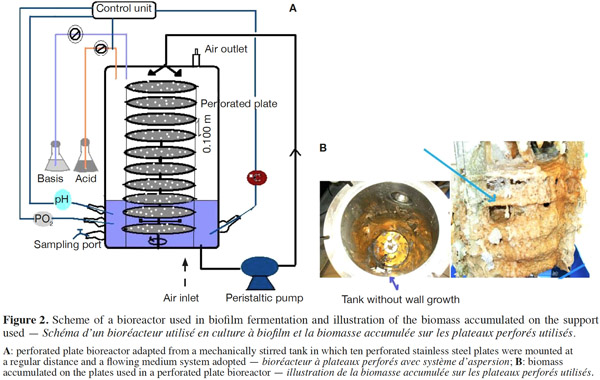
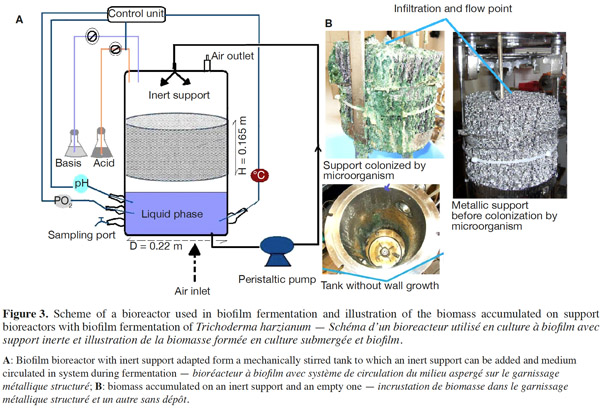
13Among cultivation systems, while solid-state fermentation exhibits a higher productivity of secondary metabolites, Kumar et al. (1987) as well as Hölker et al. (2004) compared submerged with liquid-surface fermentations and pointed to some inherent disadvantages, such as poor heat dissipation. Slow diffusion rates of nutrients, products, water, and oxygen in packed beds have also been reported (Viccini et al., 2001; Bellon-Maurel et al., 2003; Durand, 2003; Mitchell et al., 2003; Mo et al., 2004). Muffler et al. (2014) pointed out that the relevance of the emerging field of biofilm research in biotechnology becomes particularly obvious if one considers the number of publications per year for the terms ‘‘biofilm’’ and ‘‘biotechnology’’ collected by the Web of Knowledge (Thomson Reuters) in June 2013. Even though only a small fraction of the number of papers is directly focused on productive biofilms, the illustration shows the prosperous field of this research area.
2.4. Strategies for enhancing the production of fungal metabolites
14By considering the fungal biology found when biomass is attached onto a support, some researchers tried to develop a process by which secondary metabolites could be enhanced according to the needs of the microorganisms. Nevertheless, generally much higher yields were obtained in solid-state fermentation. They used, for example, submerged cultures in flasks and/or a stirrer tank (Gaspar et al., 1997; Gaspar et al., 1998; Vavilova et al., 2003; Seyis et al., 2005; Ahamed et al., 2008; Meshram et al., 2008), or solid-state fermentation (Bakri et al., 2003; Sandhya et al., 2005; Assamoi et al., 2008a; Assamoi et al., 2008b; Mitchell et al., 2010) to produce the compounds under study.
15Hölker et al. (2004) reported that solid-state fermentation is currently the best method for obtaining fungal conidia from aerial hyphae. The properties of conidia produced by solid-state fermentation differ distinctly from those obtained by submerged fermentation. Fungal conidia, used as biocontrol agents against fungal plant pathogens, are preferentially produced in solid-state fermentation; the conidia obtained manifest a higher quality. They are more resistant to desiccation and more stable in the dry state. To obtain a high number of conidia, a combination of submerged fermentation (for biomass production in the first step) and solid-state fermentation (for subsequent conidia production) proved to be successful. However, a direct comparison between solid-state fermentation and submerged fermentation is very difficult due to the different consistencies of the fungal cultures used in the two technologies. In this regard, biofilm cultures should represent a good objective due to the fact that aerial growth with a flowing medium, which seems to be similar to their natural development, can result in continuous high density production of secondary metabolites and conidia.
3. Applications involving Trichoderma, Penicillium and Aspergillus
16According to their capability of producing different compounds, the three genera, Trichoderma, Penicillium and Aspergillus are used in many industrial applications. These mycofactories can be used for two classes of applications i.e. either the production of secondary metabolites, or the production of conidia for biocontrol applications.
3.1. Cultivation of Trichoderma for the production of fungal metabolites
17The filamentous fungus Trichoderma has been cultivated using submerged, solid-state and biofilm fermentation in order to produce secondary metabolites as well as conidia. The molecules which have been taken into account were cyclosporin, 6-pentyl-α-pyrone, cellulase, hemicellulase and amylase.
18Vinale et al. (2008) showed that MVOCs of the fungus Trichoderma act antibiotically against pathogenic plant moulds and can confer plant growth promoting effects as well as systemic resistance to plants, thus rendering plants less vulnerable to fungal pathogens (Harman et al., 2004).
19A number of studies have been conducted to evaluate the effect of nutritional factors (Yong et al., 1986; Serrano-Carreon et al., 1992) on solid and liquid fermentation methods (Kalyani et al., 2000; Sarhy-Bagnon et al., 2000), on 6-pentyl-α-pyrone production. Particular attention has been accorded to enzymes with high xylanolytic activity produced by Trichoderma spp. (Wong et al., 1992). Various fermentation systems have been used with Trichoderma harzianum Rifai, Trichoderma viride Persoon and Trichoderma koningii Oudemans to produce these compounds, such as submerged (Cutler et al., 1986; Simon et al., 1988; Evidente et al., 2003), aqueous two-liquid-phase (Rito-Palomares et al., 2000; Rito-Palomares et al., 2001), organic-aqueous two-liquid-phase (Serrano-Carreón et al., 2002; Rocha-Valadez et al., 2006), liquid-surface (Kalyani et al., 2000), and solid-state cultivation systems (Cooney et al., 1997a; Cooney et al., 1997b).
20Shinobu et al. (2009) developed an extractive fermentation system with characteristics of both a liquid surface immobilization (LSI) system and a liquid-liquid interface bioreactor (L–L IBR). The system is tentatively named by the authors an “extractive liquid surface immobilization” (Ext-LSI) system. In the Ext-LSI system, the fungus-microsphere mat produces hydrophobic secondary metabolites using nutrients contained in a liquid medium. It is expected that the hydrophobic metabolites are spontaneously extracted from the fungal cells into an organic phase where they accumulate to high concentrations because feedback inhibition and cellular toxicity of the metabolites may be effectively alleviated. The reported maximum accumulations of 6-pentyl-α-pyrone (6PP) in submerged, liquid surface, and solid-state fermentations were 474 mg·l-1 (Serrano-Carreon et al., 2004), 455 mg·l-1 (Kalyani et al., 2000) and 3 g·kg-1 (de Araujo et al., 2002), respectively. Shinobu et al. (2009) also tried to apply the Ext-LSI system with a fungus to produce an aromatic coconut compound. Following optimization of the conditions to produce this volatile, the accumulation of the metabolite in the organic phase reached 7.1 g·l-1 during a four week cultivation. These examples proved that the production of secondary metabolites can be enhanced by using solid-state fermentation.
21Looking at the relationship between secondary metabolites and conidiation, Calvo et al. (2002) suggested three kinds of relations that can be considered:
22– metabolites that activate conidiation (for example, the linoleic acid derived compounds produced by Aspergillus nidulans);
23– pigments required for conidiation structures (for example, melanins required for the formation or integrity of both sexual and asexual conidia and overwintering bodies);
24– toxic metabolites secreted by growing colonies at the approximate time of conidiation (for example, the biosynthesis of some deleterious natural products, such as mycotoxins).
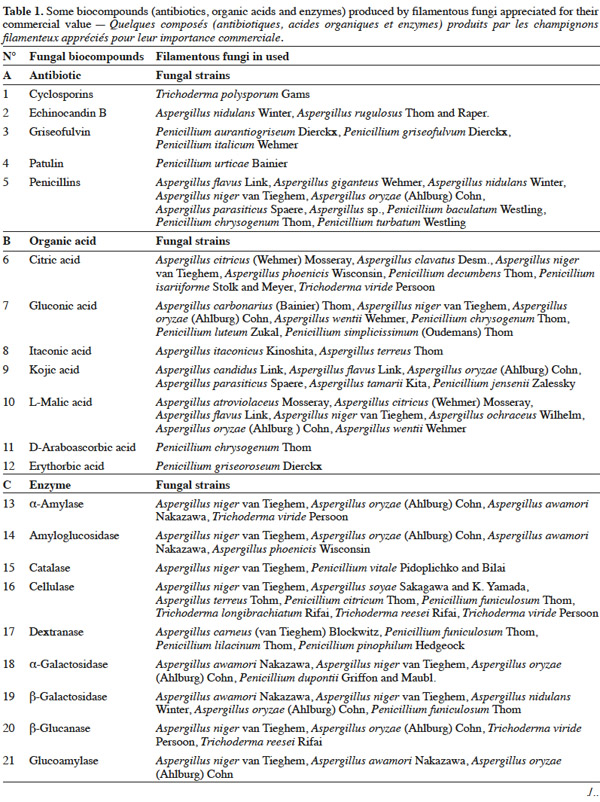
25Table 1 summarizes some examples of secondary metabolites produced by microorganisms with commercial importance, like antibiotics, organic acids and enzymes.
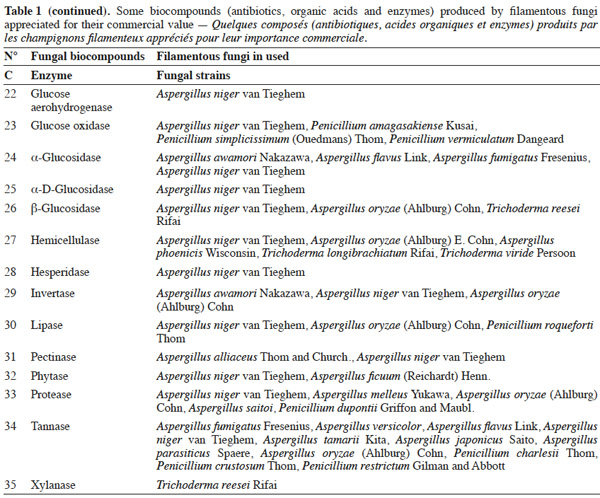
26The term ‘‘secondary metabolite’’ includes a heterogeneous group of chemically distinct natural compounds possibly related to survival functions for the producing organism such as competition (against other micro- and macroorganisms), symbiosis, metal transport, differentiation, etc. (Demain et al., 2000). Included in this group are antibiotics, which are natural products that can inhibit microbial growth. Antibiotic production is often well correlated with biocontrol ability, and the application of purified antibiotics has been found to show positive effects on the host pathogen similar to those obtained using the corresponding living microorganism. Ghisalberti et al. (1990) demonstrated that the biocontrol efficacy of Trichoderma harzianum Rifai isolates against Gaeumannomyces graminis var. ‘tritici’ is related to the production of pyrone like antibiotics.
27Trichoderma spp. produces a plethora of secondary metabolites with biological activity (Ghisalberti et al., 1991; Sivasithamparam et al., 1998). The production of secondary metabolites by Trichoderma spp. is strain dependent and includes antifungal substances belonging to a variety of chemical classes that were classified by Ghisalberti et al. (1991) into three categories:
28– volatile antibiotics, for example 6-pentyl-α-pyrone and most of the isocyanide derivates;
29– water-soluble compounds, like heptelidic acid or koningic acid;
30– peptaibols, which are linear oligopeptides of 12–22 amino acids rich in α-aminoisobutyric acid, N-acetylated at the N-terminus and containing an amino alcohol (Pheol or Trpol) at the C-terminus (Le Doan et al., 1986; Rebuffat et al., 1989).
31The results obtained from experiments conducted by some researchers suggest that microorganisms produce secondary metabolites under different bioprocesses (Table 2). Both mutant and wild strains were used. In some cases, a mutant gave a higher production than the wild strain. The particular bioprocess used also had an impact on enhancing the production.
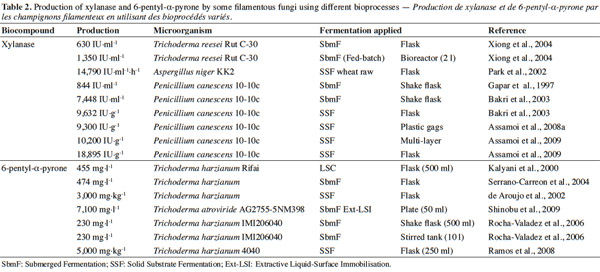
32Polizeli et al. (2005) reported that in either submerged or solid-state fermentation could be used to generate xylanase production by microorganisms. For example, submerged fermentation was used to produce xylanase from T. reesei. In that experiment, the optimal pH and temperature were 4.5 and 40 °C. This enzyme is usually used in cellulose pulp bleaching and animal feed.
33Dashtban et al. (2011) performed experiments relating to the effect of different carbon sources on cellulose production by Hypocrea jecorina (T. reesei) strains using T. reesei QM6a, T. reesei QM9414, and T. reesei RUT-C30. Time course experiments showed that maximum cellulose activity with QM6a and QM9414 strains occurred at 120 h for the majority of the tested carbon sources, while RUT-C30 had its greatest cellulose activity at around 72 h. Maximum cellulase production was observed to be 0.035, 0.42 and 0.33 μmol glucose equivalents using microcrystalline celluloses for QM6a, QM9414, and RUTC-30, respectively; while the maximum dry weight for all three T. reesei strains was obtained at the highest carbon concentration level (2%). This suggests that an increase in enzyme production is directly caused by an increase in mycelium density and may be a result of complex inducing factors in a relationship with the bioreactor configuration used.
34In the course of experiments carried out in our laboratory, it was observed that during culture, wall growth of the fungi is amplified by the speed of agitation, which splashes some fragments of mycelia on the wall vessel (Figure 1B). This phenomenon reduces the biomass in that type of culture. In order to perform the production of biomass and the volatile, a biofilm bioreactor has been adapted, modulating plate bioreactor from the mechanical one with ten perforated stainless steel plates (Figure 2B) and also using an inert metallic support named packing (Figure 3B) on which biomass can be accumulated without growth on the vessel wall. With these results, it can be suggested that the wall growth in submerged culture will be moderated using the strategies adopted.
35The similar system has been used by Khalesi et al. (2014) for the production of hydrophobin HFBII by T. reesei. They noted that the use of a biofilm reactor has led to a significant increase of HFBII production in shorter time by comparison with a classical submerged bioreactor.
3.2. Use of Penicillium for the production of fungal metabolites and conidia
36The main molecules produced by Penicillium were griseofluvin, penicillin, citric acid, koji acid, erythorbic acid, catalase, dextranase and tamase. Chávez et al. (2005) reviewed the genus Penicillium, particularly the species producing xylanase. They reported that the Penicillia were mostly saprophytic in nature, and numerous species are of particular value for humans. The best known are Penicillium canescens 10-10c and Penicillium notatum Westling, producing xylanase and the antibiotic penicillin, respectively, as well as Penicillium roqueforti Thom and Penicillium camemberti Thom, which are important in the food industry. The latter are associated with the production of particular types of cheese. A lot of Penicillia are soil fungi, and grow in a variety of organic substances, particularly dead plant materials. They produce extracellular hydrolases such as pectic enzymes, lipases, proteases, cellulases and xylanases. The latter enzymes are known for a number of biotechnological applications. One important use in cellulose pulp biobleaching (Polizeli et al., 2005) is to eliminate lignin residues from kraft pulp. They also increase the digestibility of feed by lowering the viscosity in the intestinal tract, thus improving nutrient uptake (Twomey et al., 2003). In baking, they are added to increase the specific volume and in this way improve final flavour (Maat et al., 1992). In beer and juice processing, they are used to reduce haze formation by solubilising long chain arabinoxylans (Dervilly et al., 2002).
37In studies carried out with P. canescens 10-10c, it was concluded that the production of xylanase is influenced by the process used in its production, whether submerged or solid-state fermentation. The researchers who conducted the experiments in different types of fermentation noted the influence of the solid-state fermentation (9,632 IU·g-1) (Gaspar et al., 1997; Bakri et al., 2003), or solid-state fermentation with a multi-layer system (10,200 UI·g-1) (Assamoi et al., 2008a; Assamoi et al., 2008b; Assamoi et al., 2009). In order to improve oxygen transfer in P. canescens 10-10c culture, Gaspar et al. (1997) studied the influence of the agitation and aeration rates on KLa in a mono-agitated reactor; the authors concluded that a reduction in specific power is more profitable to xylanase production than an increase in KLa beyond a critical value. Too little KLa is harmful to biomass growth. This was verified when production at 200 rpm with DT4 turbine (P = 102 W·m-3, KLa at about 20 h-1) reached only 370 IU·ml-1. An optimum was found at 300 rpm with FBT8 mobile (P = about 232 W·m-3, KLa at about 62 h-1), where production reached 844 IU·ml-1. Bakri et al. (2003) performed experiments in order to evaluate the influence of carbon sources, nitrogen sources, and moisture content on xylanase production by P. canescens 10-10c in solid-state fermentation. The experiments started in submerged cultures using Erlenmeyer flasks (250 ml) containing 50 ml of the medium; other experiments were conducted using solid-state culture. The maximum enzyme production obtained was 7,448 IU·g-1 in submerged culture and 9,632 IU·g-1 in solid-state culture after 12 days. The yields of xylanase productivity from P. canescens 10-10c observed were approximately 1.5 fold higher than optimum productivities reported in the literature for some microorganisms grown in solid-state fermentation. According to the results from Assamoi et al. (2008a), Assamoi et al. (2008b), and Assamoi et al. (2009), xylanase can be produced in solid-state culture. The experiments were carried out either in Erlenmeyer flasks or reactors with P. canescens 10-10c. In flasks, it was observed that production was maximal after seven days, 18,895 IU·g-1. Thereafter, the authors carried out a scale-up in plastic bags and a multi-layer reactor. The results after seven days were 9,300 IU·g-1 in plastic bags and 10,200 IU·g-1 in the multi-layer reactor. These results demonstrated that many problems occur in solid-state fermentation as the scale of the process increases; in particular, transport phenomena are strongly affected by the scale of the process. In this case, oxygen transfer inside the plastic bags is expected to be very low. In order to improve the oxygen transfer effectively, it would be interesting, for example, to supplement the plastic bags with a softened forced aeration (low and intermittent humidified air flow rate). Polizeli et al. (2005) summarized data on various commercial xylanases produced in submerged and solid-state fermentation cultures by microorganisms. It should be noted that about 80-90% of all xylanases are produced in submerged culture. Wheat bran and rice are regarded as inducers.
3.3. Secondary metabolites produced by Aspergillus in different bioreactor designs
38The fungal metabolites produced by Aspergillus were penicillin, citric acid, koji acid, L_malic acid, amylase, catalase, cellulase, galactosidase, glucanase, glucosidase, hemicellulase, lipase, pectinase and protease. de Vries et al. (2001) reported that within the genus Aspergillus, comprising a group of filamentous fungi with a large number of species, the most important for industrial applications are some members of the group of black Aspergilli (Aspergillus niger van Tieghem and Aspergillus tubingensis [Schöber] Mosseray). Reclassification using molecular and biochemical techniques resulted in clear distinctions being made between eight groups of black Aspergilli: A. niger, A. tubingensis, Aspergillus foetidus (Nakazawa) Thom and Rapper, Aspergillus carbonarius (Bainier) Thom, Aspergillus japonicus Saito, Aspergillus aculeatus Iizuka, Aspergillus heteromorphus Batista and Maia, and Aspergillus ellipticus Raper and Fennell. Products of several of these species have attained a generally recognized as safe (GRAS) status, which allows them to be used in food and feed applications. Black Aspergilli have a number of characteristics that make them ideal organisms for industrial applications, such as good fermentation capabilities and high levels in protein secretion. The results obtained in a comparative study on protease yield by solid-state fermentation or submerged fermentation indicated that among the various agro-industrial residues used, wheat bran was the most suitable substrate for protease synthesis by A. oryzea NRRL 1808 in submerged culture as well as solid-state fermentation. The solid-state fermentation showed its superiority for enzyme production over submerged culture, since a comparative evaluation of protease yields by the two fermentation systems showed 3.5-fold greater enzyme production using the solid-state format (Sandhya et al., 2005). A mixture of rice bran, rice husk and gram hull in 5:3:2 ratio was found to be the most suitable, where maximum enzyme production occurred after 72 h and 96 h of incubation under submerged and solid-state fermentation conditions, respectively (Nehra et al., 2002). Production of xylanase by the A. niger KK2 mutant in solid-state fermentation reached 14,790 IU· l-1·h-1 (Park et al., 2002); the authors remarked during experimentation that the highest production of xylanase (2,596 IU·gds-1) was obtained at 40 °C after six days; at higher temperatures, its production decreased sharply, suggesting that the end point of fermentation should be carefully controlled because synthesized xylanase could be degraded by non-specific proteases secreted by the fungus; the maximum value of xylanase activity achieved was 5,071 IU·g-1 of rice straw, which is similar to the 5,484 IU·g-1 of rice straw predicted by the model proposed. Wang et al. (2005) reported that protease could be produced from Aspergillus oryzea (Ahlburg) Cohn by either solid-state or submerged fermentation. However, problems exist in both processes. Besides incomplete nutrient utilization and frequent bacterial contamination, solid-state fermentation also suffers from difficulties in establishing a constant moisture content, evenly distributed aeration and effective removal of metabolic heat. In submerged fermentation, filamentous fungi exhibit various morphologies, ranging from dispersed mycelia to pellets. In some cases, dispersed growth benefits enzyme production, but in a medium of low viscosity; it is difficult to achieve absolutely dispersed mycelia, although fungal morphology varies with broth pH, inoculum size and quality, dissolved oxygen tension, mixing intensity, polymer additives and surfactant addition. For the production of xylanase, Beg et al. (2001) reported that the advantages of solid-state fermentation processes over liquid-batch fermentation include smaller volumes of liquid required for product recovery, cheap substrate, low cultivation cost for fermentation, and low risk of contamination in addition to high enzyme yield. Seye et al. (2014) carried out experiments with a fungal biofilm reactor combining the technological advantages of submerged fermentation (i.e., free water facilitating the control of culture parameters) with the biological characteristics found in solid-state fermentation in order to establish effect of insecticidal activity of conidia and metabolites secreted by an entomopathogenic A. clavatus strain in this culture system against Culex quinquefasciatus larvae and adults. They concluded that the process allowed facility in recuperation and purification of conidia (confined on the solid substrate) and metabolites (contained in the liquid medium). These results confirm the useful of the system, which can be used in similar production for other fungi. Submerged fermentation is used more often than solid-state fermentation even this solid-state gives high production of biomolecules and conidia. Another bioreactor type that can enhance production of fungal metabolites and conidia in comparison with those produced in submerged culture is biofilm bioreactor which simulates solid-state. Gutiérrez-Correa et al. (2012) discussed the main biological mechanisms that support the development of filamentous fungal biofilms and the recent advances on their use in industrial processes with an emphasis on Aspergillus biofilms. They concluded that cell adhesion appears to be the trigger for biofilm formation and its main industrial features such as increased production of metabolites and enzymes. In this sense, a great deal of information has been gained relating to the industrial uses of biofilms from Aspergillus and other filamentous fungi, which were originally considered as derived from cell immobilization systems. Efforts to seek an agreement are required to differentiate filamentous fungal biofilms from those cell immobilization systems based on “natural” or “passive” forces, since this would encourage deep research to understand gene regulation systems and behaviour in such a way to achieve its optimization, scale-up, and industrial production.
4. Conclusions
39The aim of this paper was to highlight the process used for optimizing the production of biomolecules secreted by filamentous fungi. Traditional methods used for the production of enzymes and secondary metabolites have been focused on solid-state fermentation without controlling factors essential for microorganism growth. However, submerged fermentation is more widely used, considering the possibility to implement robust control loops allowing to ensure the reproducibility of the process. The cost of secondary metabolite is one of the main factors determining the economics of process. In some cases, solid-state fermentation offers advantages over submerged fermentation, but in general submerged cultures are used most often since the parameters can be controlled efficiently. As filamentous fungi are naturally adapted to grow on surfaces, under which conditions they show a particular physiological behaviour different from that observed in submerged fermentation, another bioreactor process named “biofilm” that can enhance the production of fungal metabolites using a synthetic support within a liquid environment should also be used in order to enhance the production of biomolecules needed. This biofilm reactor can be considered as a promising technology combining the advantages of submerged fermentation in terms of process control and the advantages of solid-state fermentation in terms of fungal biology and development.
40Acknowledgements
41Michel Musoni is the recipient of a grant from the Belgian Technical Cooperation (BTC/CTB).
Bibliographie
Ahamed A. & Vermette P., 2008. Enhanced enzyme production from mixed cultures of Trichoderma reesei RUT-30 and Aspergillus niger LMA grown as fed batch in stirred tank bioreactor. Biochem. Eng. J., 42, 41-46.
Assamoi A.A. et al., 2008a. Improvement of xylanase production by Penicillium canescens 10-10c in solid-state fermentation. Biotechnol. Agron. Soc. Environ., 12, 111-118.
Assamoi A.A. et al., 2008b. Solid-state fermentation of xylanase from Penicillium canescens 10-10c in multi-layer-packed bed reactor. Appl. Biochem. Biotechnol., 145, 87-98.
Assamoi A.A., Destain J. & Thonart Ph.., 2009. Aspects microbiologiques de la production par la fermentation solide des endo-β-1,4-xylanases de moisissures : le cas de Penicillium canescens. Biotechnol. Agron. Soc. Environ., 13(2), 271-280.
Bakri Y., Jacques Ph. & Thonart Ph., 2003. Xylanase production by Penicillium canescens 10-10c in solid-state fermentation. Appl. Biochem. Biotechnol., 108, 737-748.
Barrios-González J., 2012. Solid-state fermentation: physiology of solid medium, its molecular basis and applications. Process Biochem., 47, 175-185
Beg Q.K., Kapoor M., Mahajan L. & Hoondal G.S., 2001. Microbial xylanases and their industrial applications: a review. Appl. Environ. Microbiol., 56, 326-338.
Bellon-Maurel V., Orliac O. & Christen P., 2003. Sensors and measurements in solid-state fermentation: a review. Process Biochem., 38, 881-896.
Calvo A.M., Wilson A.R., Bok J.W. & Keller N.P., 2002. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev., 66(3), 447-459.
Chávez R., Bull P. & Eyzaguirre J., 2005. The xylanolytic enzyme system from the genus Penicillium. J. Bacteriol., 123, 413-433.
Cooney J.M., Lauren D.R., Jensen D.J. & Perry-Meyer L.J., 1997a. Effect of harvest time, temperature, light, and spore inoculum concentration on 6-n-pentyl-2H-pyran-2-one production by Trichoderma spp. J. Agric. Food Chem., 45, 2802-2806.
Cooney J.M., Lauren D.R., JensenD.J. & Perry-Meyer L.J., 1997b. Effect of solid substrate, liquid supplement, and harvest time on 6-n-pentyl-2H-pyran-2-one (6PAP) production by Trichoderma spp. J. Agric. Food Chem., 45, 531-534.
Cutler H.G., Cox R.H., Crumley F.G. & Cole P.D., 1986. 6-Pentyl-α-pyrone from Trichoderma harzianum: its plant growth inhibitory and antimicrobial properties. Agric. Biol. Chem., 50(11), 2943-2945.
Dashtban M., Buchkowski R. & Wensheng Q., 2011. Effect of different carbon sources on cellulase production by Hypocrea jecorina (Trichoderma reesei) strains. Int. J. Biochem. Mol. Biol., 2(3), 274-286.
de Araujo A.A., Pastore G.M. & Berger R.G., 2002. Production of coconut aroma by fungi cultivation in solid-state fermentation. Appl. Biochem. Biotechnol., 99(1-3), 747-752.
de Vries R.P. & Visser J., 2001. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol. Mol. Biol. Rev., 65(4), 497-522.
Demain A.L. & Fang A., 2000. The natural functions of secondary metabolites. Adv. Biochem. Eng. Biotechnol., 69, 31-39.
Dervilly G. et al., 2002. Isolation and characterization of high molar mass water soluble arabinoxylans from barley and barley malt. Carbohydr. Polym., 47, 143-149.
Durand A., 2003. Bioreactor designs for solid-state fermentation. Biochem. Eng. J., 13, 113-125.
Evidente A. et al., 2003. Viridepyronone a new antifungal 6-substituted 2H-pyran-2-one produced by Trichoderma viride. J. Agric. Food Chem., 51, 6957-6960.
Gaspar A., Cosson T., Roques C. & Thonart Ph., 1997. Study on the production of xylanolytic complex from Penicillium canescens 10-10c. Appl. Biochem. Biotechnol., 67, 45-58.
Gaspar A., Strodiot L. & Thonart Ph., 1998. Improvement of oxygen transfer coefficient during Penicillium canescens 10-10c culture. Appl. Biochem. Biotechnol., 70-72, 535-545.
Ghisalberti E.L., Narbey M.J., Dewan M.M. & Sivasithamparam K., 1990. Variability among strains of Trichoderma harzianum in their ability to reduce take-all and to produce pyrones. Plant Soil, 121, 287-291.
Ghisalberti E.L. & Sivasithamparam K., 1991. Antifungal antibiotics produced by Trichoderma spp. Soil Biol. Biochem., 23, 1011-1020.
Gutiérrez-Correa M., Ludena Y., Ramage G. & Villena G.K., 2012. Recent advances on filamentous fungal biofilms for industrial uses. Appl. Biochem. Biotechnol., 167(5), 1235-1253.
Harman G.E. et al., 2004. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol., 2, 43-56.
Hölker U., Höfer M. & Lenz J., 2004. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl. Microbiol. Biotechnol., 64, 175-186.
Kalyani A., Prapulla S. & Karanth N., 2000. Study of production of 6-pentyl-α-pyrone using two methods of fermentation. Appl. Microbiol. Biotechnol., 53, 610-612.
Khalesi M. et al., 2014. Fungal biofilm reactor improves the productivity of hydrophobin HFBII. Biochem. Eng. J., 88, 171-178.
Kumar P.K.R. & Lonsane B.K., 1987. Gibberellic acid by solid state fermentation: consistent and improved yields. Biotechnol. Bioeng., 30, 267-271.
Le Doan T. et al., 1986. Fluoresce in studies on the interaction of trichorzianine A IIIc with model membranes. Biochim. Biophys. Acta, 858, 1-5.
Maat J. et al., 1992. Xylanases and their application in bakery. In: Visser J., Beldam G., vanSomeren M.A.K. & Voragen A.G.J. Xylan and Xylanases. Amsterdam, The Netherlands: Elsevier, 349-360.
Meshram M. et al., 2008. Optimal xylanase production using Penicilium janthinellum NCIM 1169: a model based approach. Biochem. Eng. J., 40, 348-356.
Mitchell D.A., vonMeien O.F. & Krieger N., 2003. Recent developments in modeling of solid-state fermentation: heat and mass transfer in bioreactors. Biochem. Eng. J., 13, 137-147.
Mitchell D.A. et al., 2010. A model-based investigation of the potential advantages of multi-layer packed beds in solid-state fermentation. Biochem. Eng. J., 48, 195-203.
Mo H., Zhang X. & Li Z., 2004. Control of gas phase for enhanced cellulase production by Penicillium decumbens in solid-state culture. Process Biochem., 39, 1293-1297.
Muffler K. et al., 2014. Application of biofilm reactors in white biotechnology. Adv. Biochem. Eng. Biotechnol., 146, 123-161.
Nehra K.S., Dhillon S., Chaudhary K. & Singh R., 2002. Production of alkaline protease by Aspergillus sp. under submerged and solid substrate fermentation. Ind. J. Microbiol., 42(1), 43-47.
Papagianni M., 2004. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv., 22, 189-259.
Park Y.S., Kang S.W., Lee J.S. & Hong S.I., 2002. Xylanase production in solid-state fermentation by Aspergillus niger mutant using statistical experimental designs. Appl. Microbiol. Biotechnol., 58, 761-766.
Polizeli M.L.T.M. et al., 2005. Xylanases from fungi: properties and industrial applications. Appl. Biochem. Biotechnol., 67, 577-591.
Rebuffat S. et al., 1989. Isolation, sequence and conformation of seven trichorzianines B from Trichoderma harzianum. Int. J. Pept. Protein Res., 34, 200-210.
Rito-Palomares M., Negrete A., Galindo E. & Serrano-Carreon L., 2000. Aroma compounds recovery from mycelial cultures in aqueous two-phase process. J. Chromatogr. B, 743, 403-408.
Rito-Palomares M. et al., 2001. The potential application of aqueous two-phase systems for in situ recovery of 6-pentyl-infinity-pyrone produced by Trichoderma harzianum. Enzyme Microb. Technol., 28, 625-631.
Rocha-Valadez J.A., Estrada M., Galindo E. & Serrano-Carreon L., 2006. From shake flasks to stirred fermentors: scale-up of an extractive fermentation process for 6-pentyl-α-pyrone production by Trichoderma harzianum using volumetric power input. Process Biochem., 41(6), 1347-1352.
Sandhya C., Sumantha A., Szakacs G. & Pandey A., 2005. Comparative evaluation of neutral protease production by Aspergillus oryzae in submerged and solid-state fermentation. Proc. Biochem., 40, 2689-2694.
Sarhy-Bagnon V., Lozano P., Saucedo-Castañeda G. & Roussos S., 2000. Production of 6-pentyl-α-pyrone by Trichoderma harzianum in liquid and solid cultures. Process Biochem., 36, 103-109.
Serrano-Carreon L., Hathout Y., Bensoussan M. & Belin J.M., 1992. Production of 6-pentyl-a-pyrone by Trichoderma harzianum from 18:n fatty acid methyl esters. Biotechnol. Lett., 14(11), 1019-1024.
Serrano-Carreon L., Balderas-Ruiz K., Galindo E. & Rito-Palomares M., 2002. Production and biotransformation of 6-pentyl-α-pyrone by Trichoderma harzianum in two-phase culture systems. Appl. Microbiol. Biotechnol., 58, 170-174.
Serrano-Carreon L., Flores C., Rodríguez B. & Galindo E., 2004. Rhizoctonia solani, an elicitor of 6-pentyl-α-pyrone production by Trichoderma harzianum in a two liquid phases, extractive fermentation system. Biotechnol. Lett., 26(18), 1403-1406.
Seye F. et al., 2014. Pathogenicity of Aspergillus clavatus produced in a fungal biofilm bioreactor toward Culex quinquefasciatus (Diptera: Culicidae). J. Pestic. Sci., 39(3), 127-132.
Seyis I. & Aksoz N., 2005. Xylanase production from Trichoderma harzianum 1073 D3 with alternative carbon and nitrogen sources. Food Technol. Biotechnol., 43(1), 37-40.
Shinobu O., Kunio I. & Shinichi O., 2009. Production of 6-pentyl-α-pyrone with Trichoderma atroviride and its mutant in a novel extractive liquid-surface immobilization (Ext-LSI) system. Process Biochem., 44(6), 625-630.
Simon A., Dunlop R.W., Ghisalberti E.L. & Sivasithmparam K., 1988. Trichoderma koningii produces a pyrone compound with antibiotic properties. Soil Biol. Biochem., 20, 263-264.
Sivasithamparam K. & Ghisalberti E.L., 1998. Secondary metabolism in Trichoderma and Gliocladium. In: Harman G.E. & Kubicek C.P., eds. Trichoderma and Gliocladium. London: Taylor and Francis Ltd., 139-191.
Twomey L.N. et al., 2003. The effects of increasing levels of soluble non-starch polysaccharides and inclusion of feed enzymes in dog diets on faecal quality and digestibility. Anim. Feed Sci. Technol., 108, 71-82.
Vavilova E.A., Antonova S.V., Barsukov E.D. & Vinetskii Y.P., 2003. Mechanism of overproduction of secreted enzymes in the mycelial fungus Penicillium canescens. Appl. Biochem. Microbiol., 39(3), 249-256.
Viccini G. et al., 2001. Analysis of growth kinetic profiles in solid-state fermentation. Food Technol. Biotechnol., 39(4), 271-294.
Vinale F. et al., 2008. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol., 72, 80-86.
Wang R., Law R.C.S. & Webb C., 2005. Protease production and conidiation by Aspergillus oryzea in flour fermentation. Process Biochem., 40, 217-227.
Wong K.K.Y. & Shaddler J.N., 1992. Trichoderma xylanases, their properties and application. Crit. Rev. Biotechnol., 12, 413-435.
Yong F.M. & Lim G., 1986. Effect of carbon source on aroma production by Trichoderma viride. MIRCEN J., 2, 483-488.





